Consensus Whole Mouse Brain #2: 10X gene expression#
In the previous notebook, we explored the integrated taxonomy and combined it with cell level and other metadata. In this notebook, we load and explore gene expression data, loading the expression of specific genes. We plot these expressions in the heatmaps, comparing the expression across the taxonomy and anatomical region, as well as show the gene expression in the UMAP.
%matplotlib inline
import pandas as pd
import numpy as np
from pathlib import Path
import matplotlib.pyplot as plt
from typing import List, Optional, Tuple
from abc_atlas_access.abc_atlas_cache.abc_project_cache import AbcProjectCache
from abc_atlas_access.abc_atlas_cache.anndata_utils import get_gene_data
We will interact with the data using the AbcProjectCache. This cache object tracks which data has been downloaded and serves the path to the requested data on disk. For metadata, the cache can also directly serve up a Pandas DataFrame. See the getting_started notebook for more details on using the cache including installing it if it has not already been.
Change the download_base variable to where you would like to download the data to your system.
download_base = Path('../../data/abc_atlas')
abc_cache = AbcProjectCache.from_cache_dir(
download_base
)
abc_cache.current_manifest
'releases/20251031/manifest.json'
Below we create the expanded cell metadata as was done previously in the cluster annotation tutorial.
cell_to_cluster_membership = abc_cache.get_metadata_dataframe(
'Consensus-WMB-integrated-taxonomy', 'cell_to_cluster_membership'
).set_index('cell_label')
cluster = abc_cache.get_metadata_dataframe(
'Consensus-WMB-integrated-taxonomy', 'cluster'
).set_index('label')
cluster_annotation_term = abc_cache.get_metadata_dataframe(
'Consensus-WMB-integrated-taxonomy', 'cluster_annotation_term'
).set_index('label')
cluster_annotation_term_set = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-integrated-taxonomy',
file_name='cluster_annotation_term_set'
).set_index('label')
cluster_to_cluster_annotation_membership = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-integrated-taxonomy',
file_name='cluster_to_cluster_annotation_membership'
).set_index('cluster_annotation_term_label')
macosko_cell_metadata = abc_cache.get_metadata_dataframe('Consensus-WMB-Macosko-10X', 'cell_metadata').set_index('cell_label')
aibs_cell_metadata = abc_cache.get_metadata_dataframe('Consensus-WMB-AIBS-10X', 'cell_metadata').set_index('cell_label')
membership_with_cluster_info = cluster_to_cluster_annotation_membership.join(
cluster.reset_index().set_index('cluster_alias')[['number_of_cells']],
on='cluster_alias'
)
membership_with_cluster_info = membership_with_cluster_info.join(cluster_annotation_term, rsuffix='_anno_term').reset_index()
membership_groupby = membership_with_cluster_info.groupby(
['cluster_alias', 'cluster_annotation_term_set_name']
)
cluster_details = membership_groupby['cluster_annotation_term_name'].first().unstack()
cluster_order = membership_groupby['term_order'].first().unstack()
cluster_order.sort_values(['neighborhood', 'class', 'subclass', 'supertype', 'cluster'], inplace=True)
cluster_colors = membership_groupby['color_hex_triplet'].first().unstack()
cluster_colors = cluster_colors[cluster_annotation_term_set['name']]
cluster_colors.sort_values(
['neighborhood', 'class', 'subclass', 'supertype', 'cluster'],
inplace=True
)
aibs_cell_extended = aibs_cell_metadata.join(cell_to_cluster_membership, how='inner')
aibs_cell_extended = aibs_cell_extended.join(cluster_details, on='cluster_alias')
aibs_cell_extended = aibs_cell_extended.join(cluster_colors, on='cluster_alias', rsuffix='_color')
aibs_cell_extended = aibs_cell_extended.join(cluster_order, on='cluster_alias', rsuffix='_order')
aibs_library = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-AIBS-10X',
file_name='library'
).set_index('library_label')
aibs_donor = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-AIBS-10X',
file_name='donor'
).set_index('donor_label')
aibs_cell_extended = aibs_cell_extended.join(aibs_library, on='library_label')
aibs_cell_extended = aibs_cell_extended.join(aibs_donor, on='donor_label')
macosko_cell_extended = macosko_cell_metadata.join(cell_to_cluster_membership, how='inner')
macosko_cell_extended = macosko_cell_extended.join(cluster_details, on='cluster_alias')
macosko_cell_extended = macosko_cell_extended.join(cluster_colors, on='cluster_alias', rsuffix='_color')
macosko_cell_extended = macosko_cell_extended.join(cluster_order, on='cluster_alias', rsuffix='_order')
macosko_library = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-Macosko-10X',
file_name='library'
).set_index('library_label')
macosko_donor = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-Macosko-10X',
file_name='donor'
).set_index('donor_label')
macosko_cell_extended = macosko_cell_extended.join(macosko_library, on='library_label')
macosko_cell_extended = macosko_cell_extended.join(macosko_donor, on='donor_label')
value_sets = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-AIBS-10X',
file_name='value_sets'
).set_index('label')
def extract_value_set(cell_metadata_df: pd.DataFrame, input_value_set: pd.DataFrame, input_value_set_label: str):
"""Add color and order columns to the cell metadata dataframe based on the input
value set.
Columns are added as {input_value_set_label}_color and {input_value_set_label}_order.
Parameters
----------
cell_metadata_df : pd.DataFrame
DataFrame containing cell metadata.
input_value_set : pd.DataFrame
DataFrame containing the value set information.
input_value_set_label : str
The the column name to extract color and order information for. will be added to the cell metadata.
"""
cell_metadata_df[f'{input_value_set_label}_color'] = input_value_set[
input_value_set['field'] == input_value_set_label
].loc[cell_metadata_df[input_value_set_label]]['color_hex_triplet'].values
cell_metadata_df[f'{input_value_set_label}_order'] = input_value_set[
input_value_set['field'] == input_value_set_label
].loc[cell_metadata_df[input_value_set_label]]['order'].values
# Add region of interest color and order
extract_value_set(macosko_cell_extended, value_sets, 'origin_dataset')
# Add species common name color and order
extract_value_set(macosko_cell_extended, value_sets, 'donor_sex')
# Add species scientific name color and order
extract_value_set(macosko_cell_extended, value_sets, 'anatomical_division_label')
# Add region of interest color and order
extract_value_set(aibs_cell_extended, value_sets, 'origin_dataset')
# Add species common name color and order
extract_value_set(aibs_cell_extended, value_sets, 'donor_sex')
# Add species scientific name color and order
extract_value_set(aibs_cell_extended, value_sets, 'anatomical_division_label')
Single cell and nuclei transcriptomes#
Below we use the convenience function get_gene_data to download and extract specific genes from the gene expression h5ad files. This function can be used to pull expression for the full set of cells or any subset from the set of cell metadata.
We first load the gene data for the WMB-AIBS portion of the consensus dataset. Note that this reuses the previous WMB-10X release’s genes and expression matrices.
aibs_gene = abc_cache.get_metadata_dataframe(
directory='WMB-10X',
file_name='gene'
).set_index('gene_identifier')
print("Number of aligned genes = ", len(aibs_gene))
aibs_gene.head(5)
gene.csv: 100%|██████████| 2.30M/2.30M [00:00<00:00, 3.69MMB/s]
Number of aligned genes = 32285
| gene_symbol | name | mapped_ncbi_identifier | comment | |
|---|---|---|---|---|
| gene_identifier | ||||
| ENSMUSG00000051951 | Xkr4 | X-linked Kx blood group related 4 | NCBIGene:497097 | NaN |
| ENSMUSG00000089699 | Gm1992 | predicted gene 1992 | NaN | NaN |
| ENSMUSG00000102331 | Gm19938 | predicted gene, 19938 | NaN | NaN |
| ENSMUSG00000102343 | Gm37381 | predicted gene, 37381 | NaN | NaN |
| ENSMUSG00000025900 | Rp1 | retinitis pigmentosa 1 (human) | NCBIGene:19888 | NaN |
Next we load the same metadata for the Macosko data. Note that the two samples have slightly different sets of genes, however, they have a large overlap.
macosko_gene = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-Macosko-10X',
file_name='gene'
).set_index('gene_identifier')
print("Number of aligned genes = ", len(macosko_gene))
macosko_gene.head(5)
gene.csv: 100%|██████████| 546k/546k [00:00<00:00, 2.29MMB/s]
Number of aligned genes = 21205
| gene_symbol | |
|---|---|
| gene_identifier | |
| ENSMUSG00000072572 | Slc39a2 |
| ENSMUSG00000063179 | Pstk |
| ENSMUSG00000041220 | Elovl6 |
| ENSMUSG00000042246 | Tmc7 |
| ENSMUSG00000075072 | Olfr48 |
Below we list the genes we will use in this notebook and the example method used to load the expression for these specific genes from the h5ad file. Note that we provide a set of example gene expressions as a csv file for brevity in this tutorial. To process and extract the gene expressions for yourself, uncomment the code block below. Note that there are two different calls one for the WMB-AIBS data and one for the WMB-Macosko data. These two calls need to be separated but the results can be combined afterward. Warning that this is a large amount of data and may take a significant fraction of time to download. This download action will only need to be performed once, however.
For more details on how to extract specific genes from the data see our accessing gene expression data tutorial
gene_names = ['Slc17a7', 'Slc17a6', 'Slc17a8', 'Slc32a1', 'Slc6a5', 'Slc18a3', 'Slc6a3', 'Slc6a4', 'Slc6a2']
"""
aibs_gene_data = get_gene_data(
abc_atlas_cache=abc_cache,
all_cells=aibs_cell_metadata,
all_genes=aibs_gene,
selected_genes=gene_names
)
"""
'\naibs_gene_data = get_gene_data(\n abc_atlas_cache=abc_cache,\n all_cells=aibs_cell_metadata,\n all_genes=aibs_gene,\n selected_genes=gene_names\n)\n'
"""
macosko_gene_data = get_gene_data(
abc_atlas_cache=abc_cache,
all_cells=macosko_cell_metadata,
all_genes=macosko_gene,
selected_genes=gene_names
)
"""
'\nmacosko_gene_data = get_gene_data(\n abc_atlas_cache=abc_cache,\n all_cells=macosko_cell_metadata,\n all_genes=macosko_gene,\n selected_genes=gene_names\n)\n'
Instead of processing the gene expressions, we load pre-processed files containing the expression for the genes listed above.
macosko_gene_data = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-Macosko-10X',
file_name='example_gene_expression'
).set_index('cell_label')
aibs_gene_data = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-AIBS-10X',
file_name='example_gene_expression'
).set_index('cell_label')
example_gene_expression.csv: 100%|██████████| 330M/330M [00:59<00:00, 5.51MMB/s]
example_gene_expression.csv: 100%|██████████| 291M/291M [00:53<00:00, 5.38MMB/s]
We load and merge the expression into each of our cell metadata.
aibs_cell_extended = aibs_cell_extended.join(aibs_gene_data)
macosko_cell_extended = macosko_cell_extended.join(macosko_gene_data)
Example use cases#
In this section, we show a use case plotting a set of genes that map onto a cononical set of neurotransmitters. These are the same set of genes used in the WMB-AIBS tutorial.
The helper function below creates a heatmap showing the relation between various parameters in the combined cell metadata and the genes we loaded.
import matplotlib as mpl
def plot_heatmap(
df: pd.DataFrame,
gnames: List[str],
value: str,
sort: bool = False,
fig_width: float = 8,
fig_height: float = 4,
vmax: float = None,
cmap: plt.cm = plt.cm.magma
):
"""Plot a heatmap of gene expression values for a list of genes across species.
Parameters
----------
df : pd.DataFrame
DataFrame containing cell metadata and gene expression values.
gnames : list
List of gene names to plot.
value : str
Column name in df to group by (e.g., 'species_genus').
sort : bool, optional
Whether to sort the gene expression values within each species.
fig_width : float, optional
Width of the figure in inches.
fig_height : float, optional
Height of the figure in inches.
vmax : float, optional
Maximum value for the color scale. If None, it is set to the maximum value in the data.
cmap : matplotlib colormap, optional
Colormap to use for the heatmap.
Returns
-------
fig : matplotlib.figure.Figure
The figure object containing the heatmap.
ax : array of matplotlib.axes.Axes
The axes objects for each species.
"""
fig, ax = plt.subplots(1, 1)
fig.set_size_inches(fig_width, fig_height)
grouped = df.groupby(value)[gnames].mean()
vmin = grouped.min().min()
vmax = grouped.max().max()
norm = mpl.colors.Normalize(vmin=vmin, vmax=vmax)
sm = plt.cm.ScalarMappable(cmap=cmap, norm=norm)
cmap = sm.get_cmap()
if sort:
grouped = grouped.sort_values(by=gnames[0], ascending=False)
grouped = grouped.loc[sorted(grouped.index)]
arr = grouped.to_numpy().astype('float')
ax.imshow(arr, cmap=cmap, aspect='auto', vmin=vmin, vmax=vmax)
xlabs = grouped.columns.values
ylabs = grouped.index.values
ax.set_yticks(range(len(ylabs)))
ax.set_yticklabels(ylabs)
ax.set_xticks(range(len(xlabs)))
ax.set_xticklabels(xlabs)
cbar = fig.colorbar(sm, ax=ax, orientation='vertical', fraction=0.01, pad=0.01)
cbar.set_label('Mean Expression [log2(CPM + 1)]')
plt.subplots_adjust(wspace=0.00, hspace=0.00)
return fig, ax
Average expression of selected genes#
Below we plot the expression of the genes we selected averaged across metadata terms in a heatmap. We show these by neighborhood for each experiment/dataset individually and then together. Also, we show selecting a subset of cells by their neighborhood value and both plotting them in the heatmap and an example function loading gene expression for this subset of cells only.
fig, ax = plot_heatmap(
df=aibs_cell_extended,
gnames=gene_names,
value='neighborhood',
fig_width=15,
fig_height=5,
sort=True
)
fig.suptitle('neighborhood: WMB-AIBS')
plt.show()
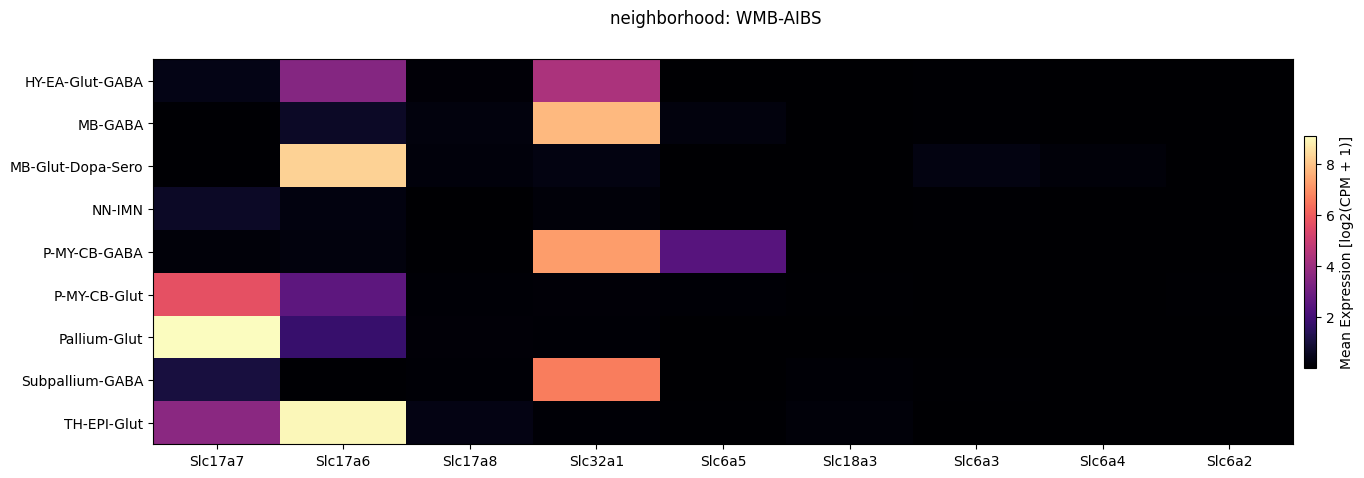
fig, ax = plot_heatmap(
df=macosko_cell_extended,
gnames=gene_names,
value='neighborhood',
fig_width=15,
fig_height=5,
sort=True
)
fig.suptitle('neighborhood: WMB-Macosko')
plt.show()
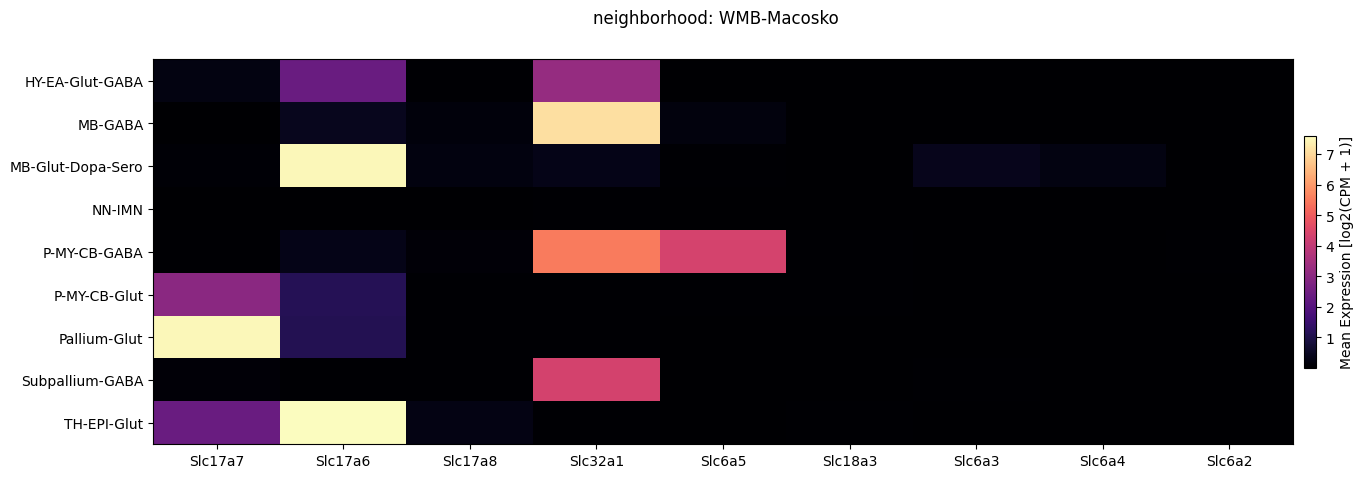
full_cell_extended = pd.concat([aibs_cell_extended, macosko_cell_extended])
fig, ax = plot_heatmap(
df=full_cell_extended,
gnames=gene_names,
value='neighborhood',
fig_width=15,
fig_height=5,
sort=True
)
fig.suptitle('neighborhood: Both')
plt.show()
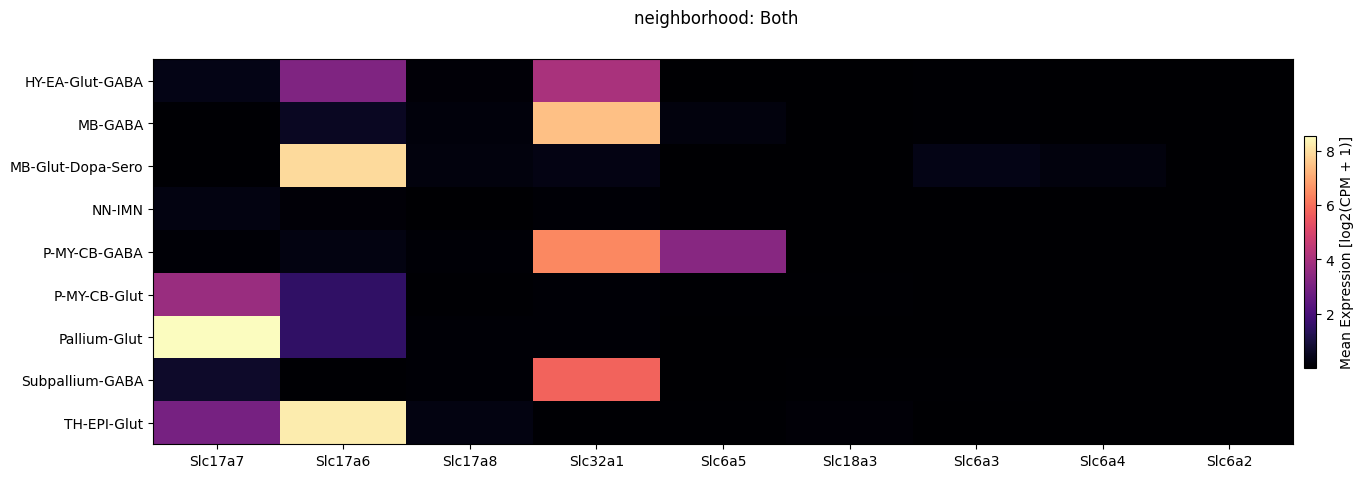
Next we show the selected genes by neurotransmitter for both datasets.
fig, ax = plot_heatmap(
df=full_cell_extended,
gnames=gene_names,
value='neurotransmitter',
fig_width=15,
fig_height=5,
sort=True
)
fig.suptitle('Neurotransmitter: Both')
plt.show()
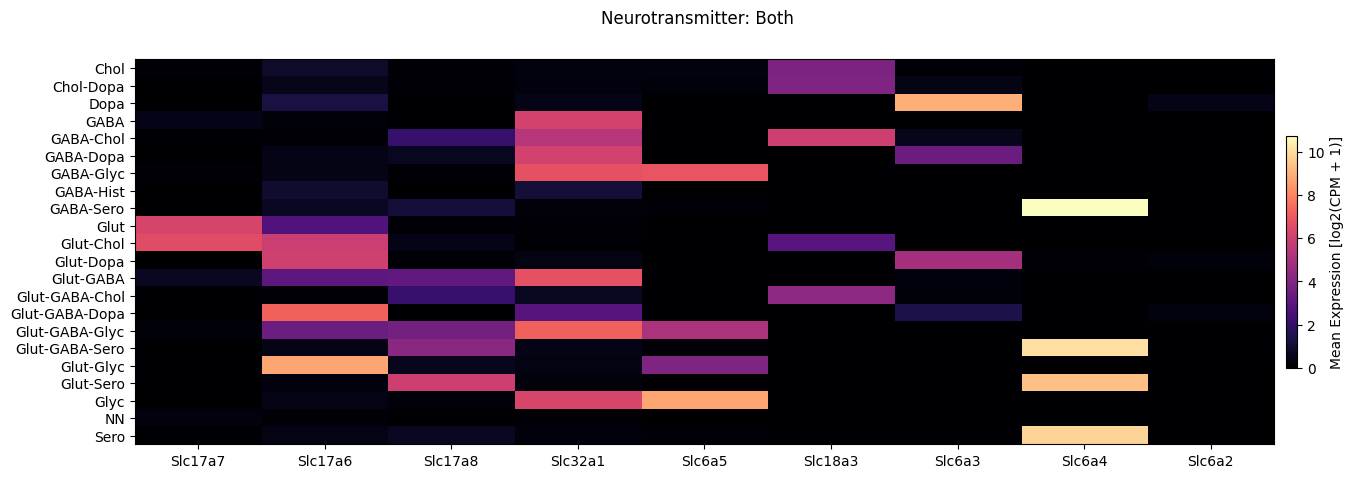
Finally, we break down the expression by anatomical region symbol.
fig, ax = plot_heatmap(
df=full_cell_extended,
gnames=gene_names,
value='anatomical_division_label',
fig_width=15,
fig_height=5,
sort=True
)
fig.suptitle('Anatomical Region: Both')
plt.show()
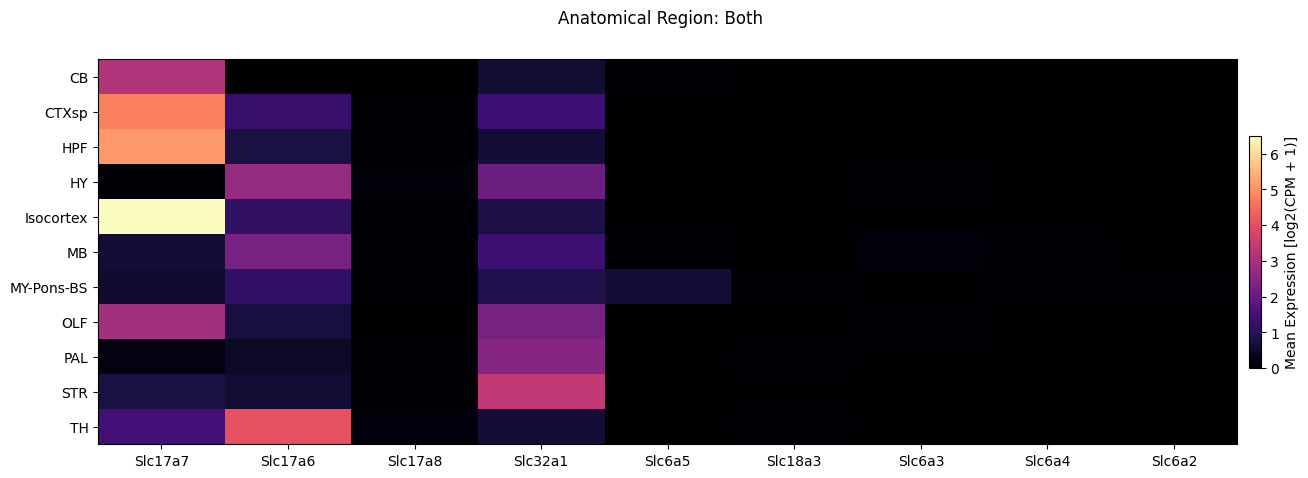
Individual neighborhood gene expression#
Next, we’ll plot the same expression vs anatomical_region, but now we’ll do so for only one of the neighborhoods, Pallium-Glut. Note that we expect the plot to be fairly dark as we are only showing the Pallium-Glut data and not all the canonical neurotransmitters will show expression.
fig, ax = plot_heatmap(
df=full_cell_extended[full_cell_extended['neighborhood'] == 'Pallium-Glut'],
gnames=gene_names,
value='anatomical_division_label',
fig_width=15,
fig_height=5,
sort=True
)
fig.suptitle('Pallium-Glut neighborhood: expression in anatomical region')
plt.show()
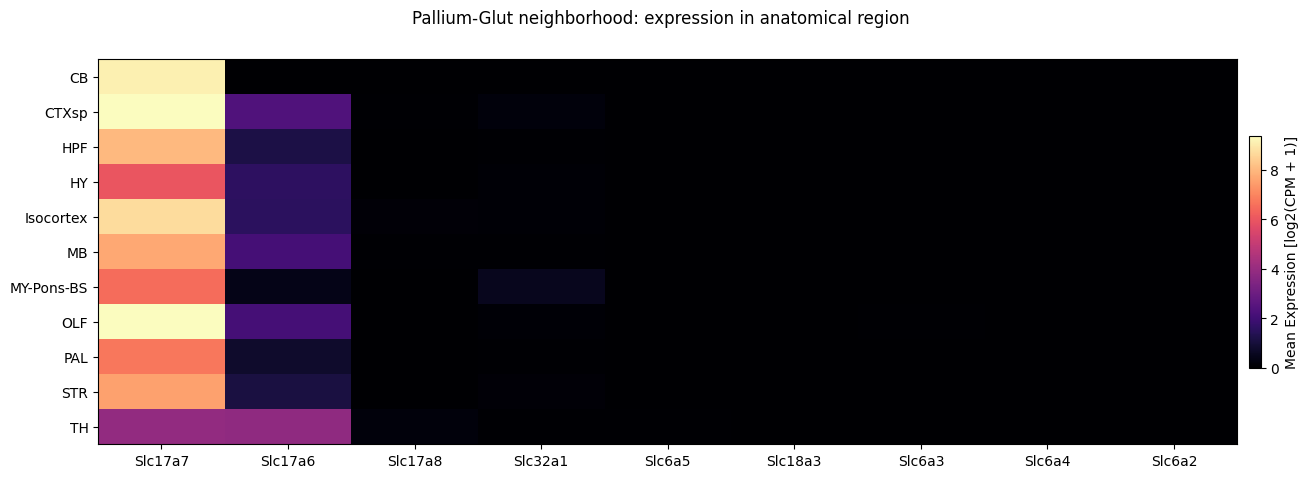
We can also directly use our cell metadata table trimmed to the selected neighborhood in the get_gene_data function to retrieve a expression for a subset of cells only. If the data is already download, this may process the data more quickly given the smaller number of cells.
# Uncomment below to for an example of how to extract gene expression for a subset of cells only
example_gene_selection = ['Elovl6']
"""
aibs_sub_cells_gene_data = get_gene_data(
abc_atlas_cache=abc_cache,
all_cells=aibs_cell_extended[aibs_cell_extended['neighborhood'] == 'Pallium-Glut'],
all_genes=aibs_gene,
selected_genes=example_gene_selection
)"""
"""
macosko_sub_cells_gene_data = get_gene_data(
abc_atlas_cache=abc_cache,
all_cells=macosko_cell_extended[macosko_cell_extended['neighborhood'] == 'Pallium-Glut'],
all_genes=macosko_gene,
selected_genes=example_gene_selection
)
"""
"\nmacosko_sub_cells_gene_data = get_gene_data(\n abc_atlas_cache=abc_cache,\n all_cells=macosko_cell_extended[macosko_cell_extended['neighborhood'] == 'Pallium-Glut'],\n all_genes=macosko_gene,\n selected_genes=example_gene_selection\n)\n"
Expression in the UMAP#
Finally, we load the UMAP coordinates for our cells and plot the expression in the UMAP for each of our selected genes.
We could also load one of the neighborhood UMAPs that we showed in the previous notebook.
cell_2d_embedding_coordinates = value_sets = abc_cache.get_metadata_dataframe(
directory='Consensus-WMB-integrated-taxonomy',
file_name='cell_2d_embedding_coordinates'
).set_index('cell_label')
cell_2d_embedding_coordinates.head()
| x | y | |
|---|---|---|
| cell_label | ||
| GCGAGAAGTTAAGGGC-410_B05 | 16.037980 | 3.101109 |
| AATGGCTCAGCTCCTT-411_B06 | 15.951514 | 3.144049 |
| AACACACGTTGCTTGA-410_B05 | 15.900673 | 3.124507 |
| CACAGATAGAGGCGGA-410_A05 | 16.062553 | 3.185574 |
| GATCGTATCGAATCCA-411_B06 | 15.971468 | 3.124298 |
Join the coordinates into the cell metadata.
consensus_extended = full_cell_extended.join(
cell_2d_embedding_coordinates
).sample(frac=1)
consensus_extended.head()
| cell_barcode | barcoded_cell_sample_label | library_label | dataset_label | feature_matrix_label | cluster_alias | class | cluster | neighborhood | neurotransmitter | ... | Slc17a7 | Slc6a5 | Slc17a6 | Slc6a2 | Slc17a8 | Slc6a4 | Slc6a3 | Slc18a3 | x | y | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cell_label | |||||||||||||||||||||
| GATCGCGTCCCTAACC-040_B01 | GATCGCGTCCCTAACC | 040_B01 | L8TX_180815_01_D08 | WMB-10Xv2 | WMB-10Xv2-TH | 10653 | 019 TH Glut | 2997 AM-IAD_Col27a1:Calb1_Glut 2 | TH-EPI-Glut | Glut | ... | 0.0 | 0.0 | 9.888697 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | -14.503504 | 5.820190 |
| pBICCNsMMrMBL5iM012d200825_CACACAAGTTCAAAGA | CACACAAGTTCAAAGA | NaN | pBICCNsMMrMBL5iM012d200825 | Consensus-WMB-Macosko-10X | Macosko-10X-MB | 13369 | 039 OPC-Oligo | 6600 OPC_NN 2 | NN-IMN | NN | ... | 0.0 | 0.0 | 0.000000 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | -6.966048 | -12.871844 |
| pBICCNsMMrCTXANGiM022d210602C1_AGGCCACCACACGGTC | AGGCCACCACACGGTC | NaN | pBICCNsMMrCTXANGiM022d210602C1 | Consensus-WMB-Macosko-10X | Macosko-10X-Isocortex | 15760 | 037 Astro-Epen | 6538 Astro-TE_NN 4 | NN-IMN | NN | ... | 0.0 | 0.0 | 0.000000 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | -14.124741 | -1.272918 |
| ATCACGAGTCATAACC-415_D01 | ATCACGAGTCATAACC | 415_D01 | L8TX_201105_01_A02 | WMB-10Xv3 | WMB-10Xv3-STR | 5188 | 009 CNU-LGE GABA | 1247 STR_D2_Gaba 2 | Subpallium-GABA | GABA | ... | 0.0 | 0.0 | 0.000000 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | -6.348766 | -2.057016 |
| pBICCNsMMrBSL4iM008d190717_GCTGCAGCACTGCGAC | GCTGCAGCACTGCGAC | NaN | pBICCNsMMrBSL4iM008d190717 | Consensus-WMB-Macosko-10X | Macosko-10X-MY-Pons-BS | 13118 | 039 OPC-Oligo | 6613 Oligo_NN MOL | NN-IMN | NN | ... | 0.0 | 0.0 | 0.000000 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | -3.101157 | -11.179463 |
5 rows × 49 columns
We use the same UMAP plotting convenience function as previously used in the Consensus WMB clustering and annotation tutorial.
def plot_umap(
xx: np.ndarray,
yy: np.ndarray,
cc: np.ndarray = None,
val: np.ndarray = None,
fig_width: float = 8,
fig_height: float = 8,
cmap: Optional[plt.Colormap] = None,
labels: np.ndarray = None,
term_orders: np.ndarray = None,
colorbar: bool = False,
sizes: np.ndarray = None,
fig: plt.Figure = None,
ax: plt.Axes = None,
) -> Tuple[plt.Figure, plt.Axes]:
"""
Plot a scatter plot of the UMAP coordinates.
Parameters
----------
xx : array-like
x-coordinates of the points to plot.
yy : array-like
y-coordinates of the points to plot.
cc : array-like, optional
colors of the points to plot. If None, the points will be colored by the values in `val`.
val : array-like, optional
values of the points to plot. If None, the points will be colored by the values in `cc`.
fig_width : float, optional
width of the figure in inches. Default is 8.
fig_height : float, optional
height of the figure in inches. Default is 8.
cmap : str, optional
colormap to use for coloring the points. If None, the points will be colored by the values in `cc`.
labels : array-like, optional
labels for the points to plot. If None, no labels will be added to the plot.
term_orders : array-like, optional
order of the labels for the legend. If None, the labels will be ordered by their appearance in `labels`.
colorbar : bool, optional
whether to add a colorbar to the plot. Default is False.
sizes : array-like, optional
sizes of the points to plot. If None, all points will have the same size.
fig : matplotlib.figure.Figure, optional
figure to plot on. If None, a new figure will be created with 1 subplot.
ax : matplotlib.axes.Axes, optional
axes to plot on. If None, a new figure will be created with 1 subplot.
"""
if sizes is None:
sizes = 1
if ax is None or fig is None:
fig, ax = plt.subplots()
fig.set_size_inches(fig_width, fig_height)
if cmap is not None:
scatt = ax.scatter(xx, yy, c=val, s=0.5, marker='.', cmap=cmap, alpha=sizes)
elif cc is not None:
scatt = ax.scatter(xx, yy, c=cc, s=0.5, marker='.', alpha=sizes)
if labels is not None:
from matplotlib.patches import Rectangle
unique_label_colors = (labels + ',' + cc).unique()
unique_labels = np.array([label_color.split(',')[0] for label_color in unique_label_colors])
unique_colors = np.array([label_color.split(',')[1] for label_color in unique_label_colors])
if term_orders is not None:
unique_order = term_orders.unique()
term_order = np.argsort(unique_order)
unique_labels = unique_labels[term_order]
unique_colors = unique_colors[term_order]
rects = []
for color in unique_colors:
rects.append(Rectangle((0, 0), 1, 1, fc=color))
legend = ax.legend(rects, unique_labels, loc=0)
# ax.add_artist(legend)
ax.set_xticks([])
ax.set_yticks([])
if colorbar:
fig.colorbar(scatt, ax=ax)
return fig, ax
Below we plot the first 2 genes in our set next to the UMAP colored by anatomical region.
term_to_plot = 'anatomical_division_label'
sub_cell_extended = consensus_extended[::10]
# Plot UMAPs for the first two genes in gene_names
for gene_name in gene_names[:2]:
fig, ax = plt.subplots(1, 2)
fig.set_size_inches(18, 9)
ax = ax.flatten()
plot_umap(
sub_cell_extended['x'],
sub_cell_extended['y'],
cc=sub_cell_extended[term_to_plot + '_color'],
labels=sub_cell_extended[term_to_plot],
term_orders=sub_cell_extended[term_to_plot + '_order'],
fig=fig,
ax=ax[0],
)
ax[0].set_title(term_to_plot)
plot_umap(
sub_cell_extended['x'],
sub_cell_extended['y'],
val=sub_cell_extended[gene_name],
cmap=plt.cm.magma,
fig=fig,
ax=ax[1],
colorbar=False,
)
ax[1].set_title(f'Gene Expression: {gene_name}')
plt.tight_layout()
plt.show()

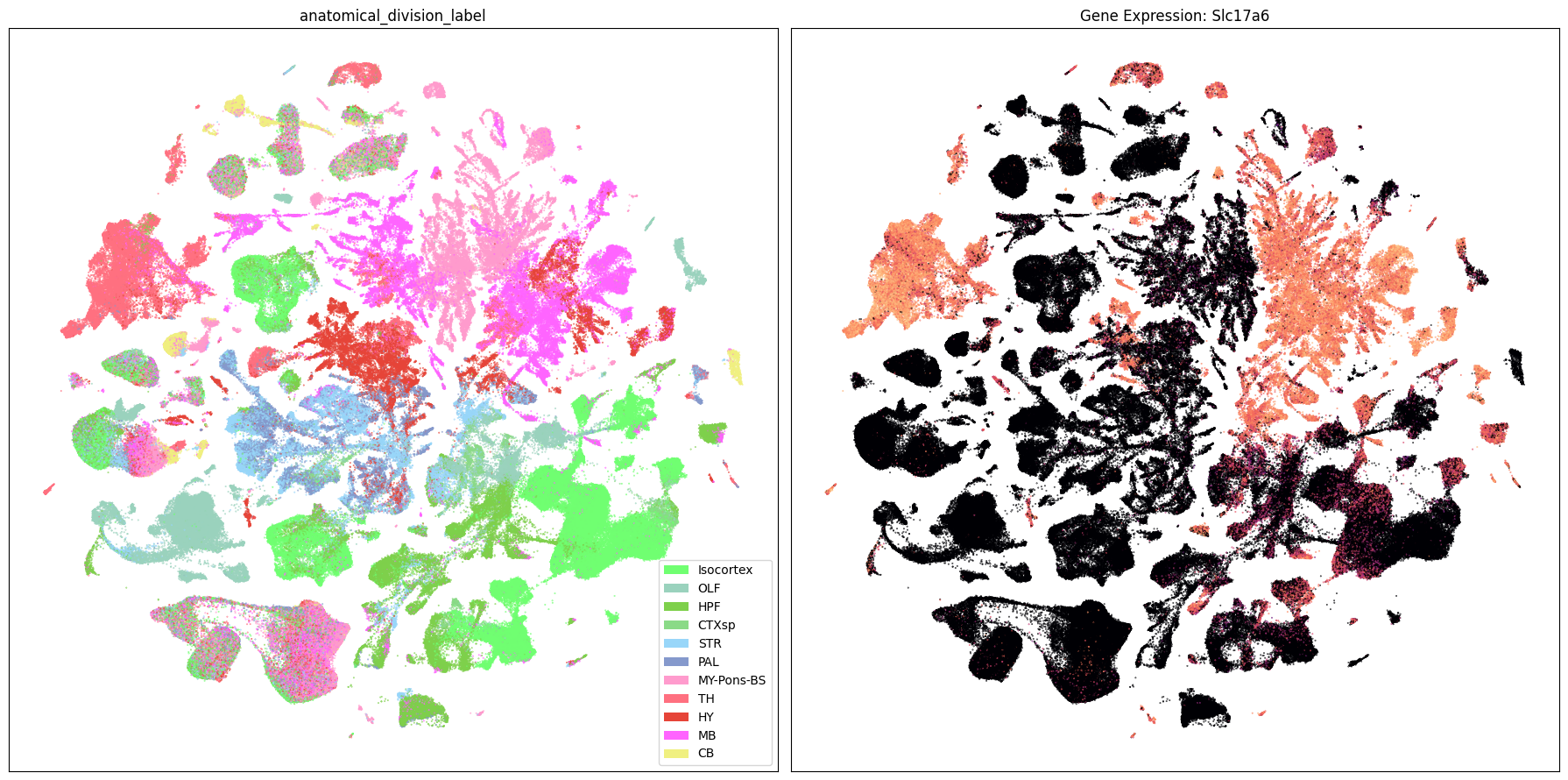
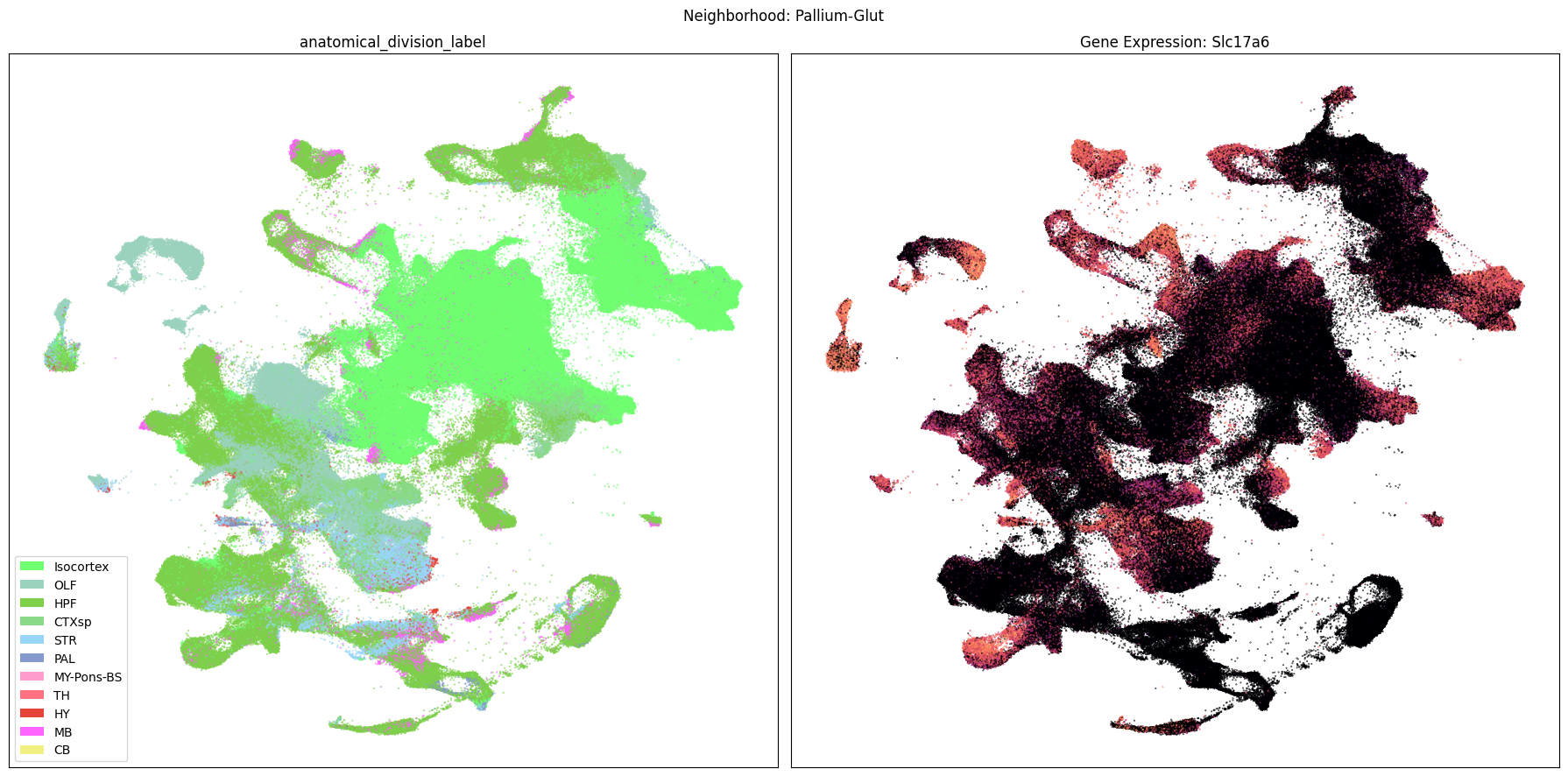
Neighborhood UMAPs#
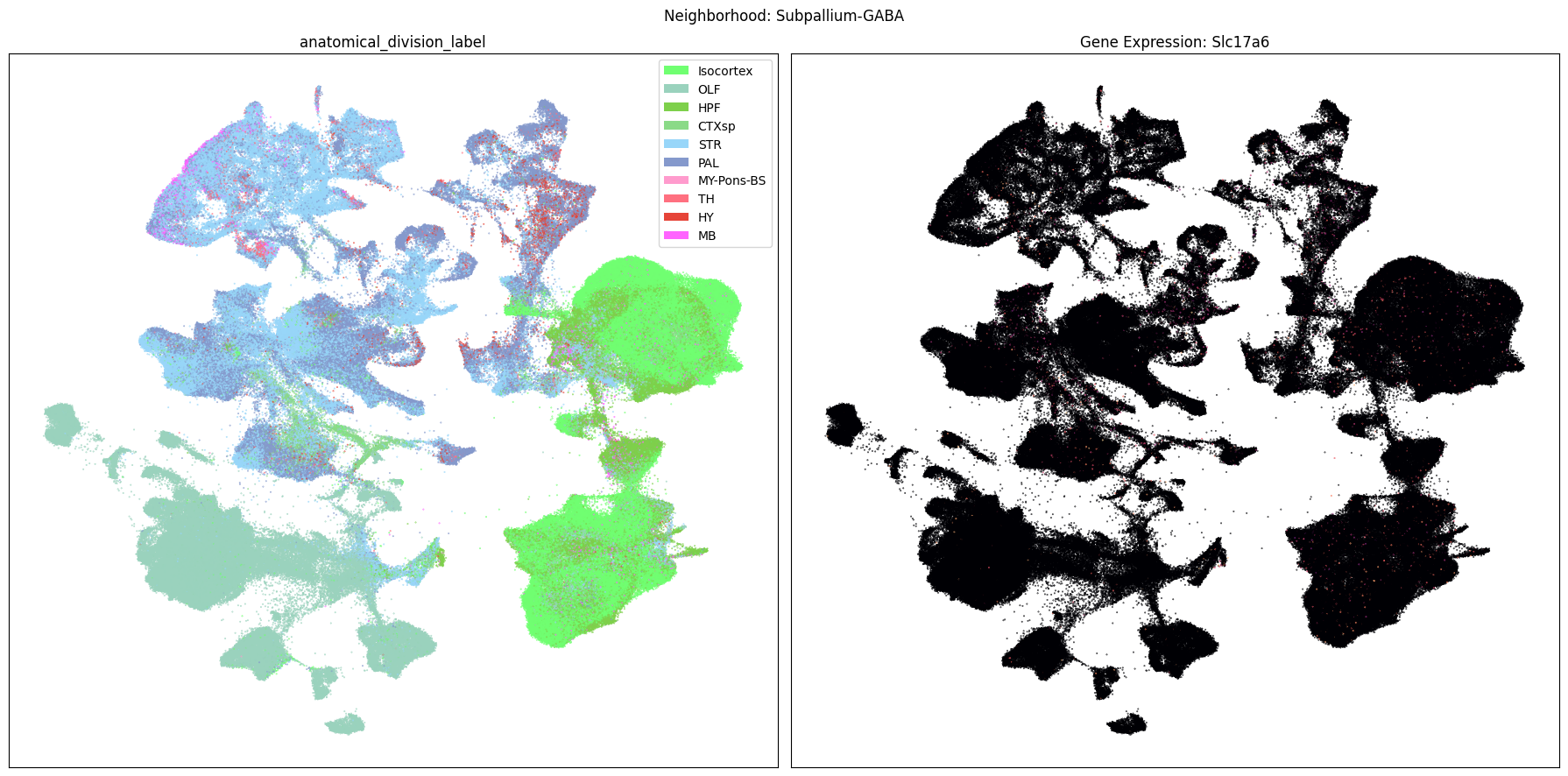
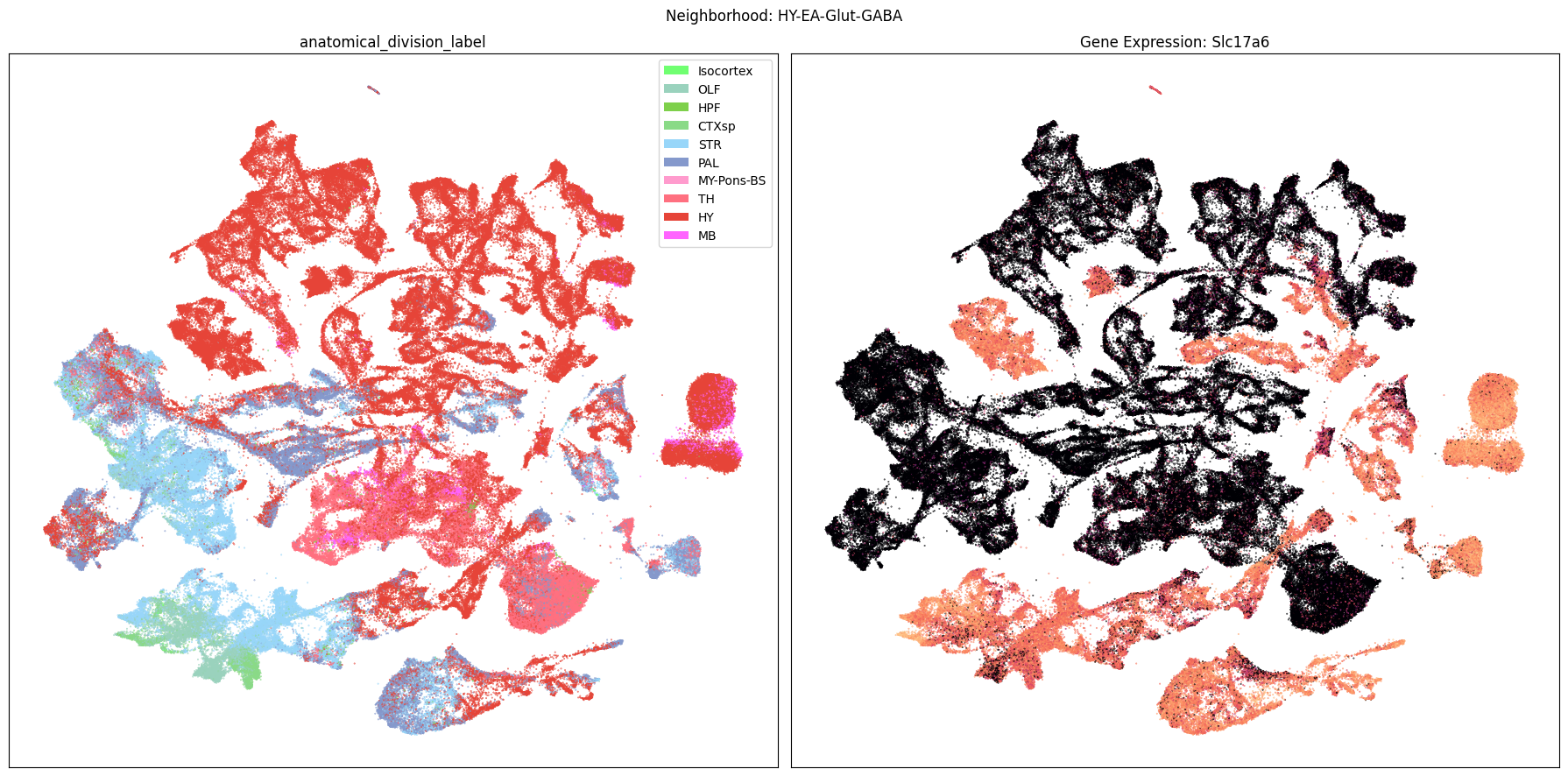
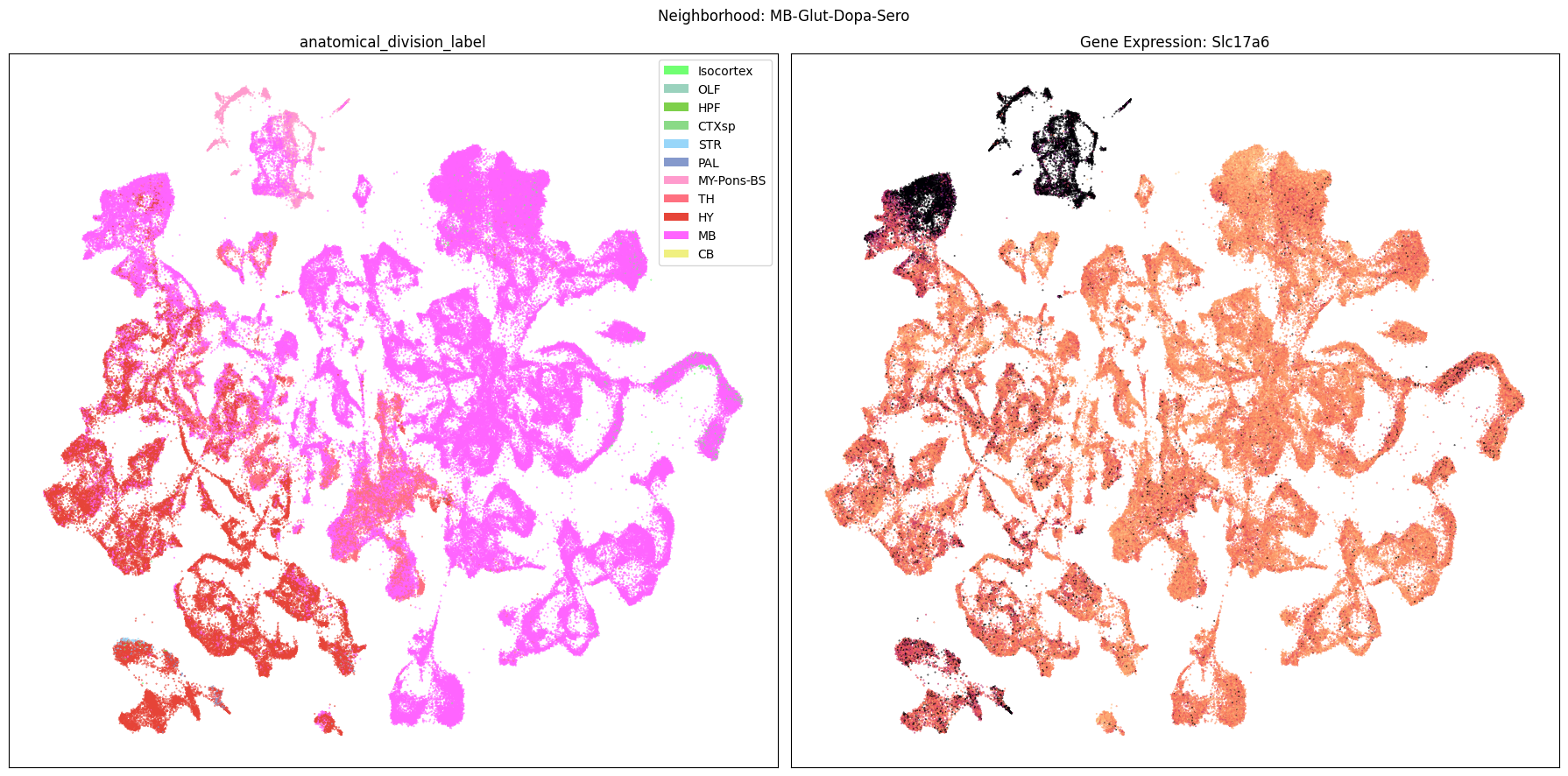
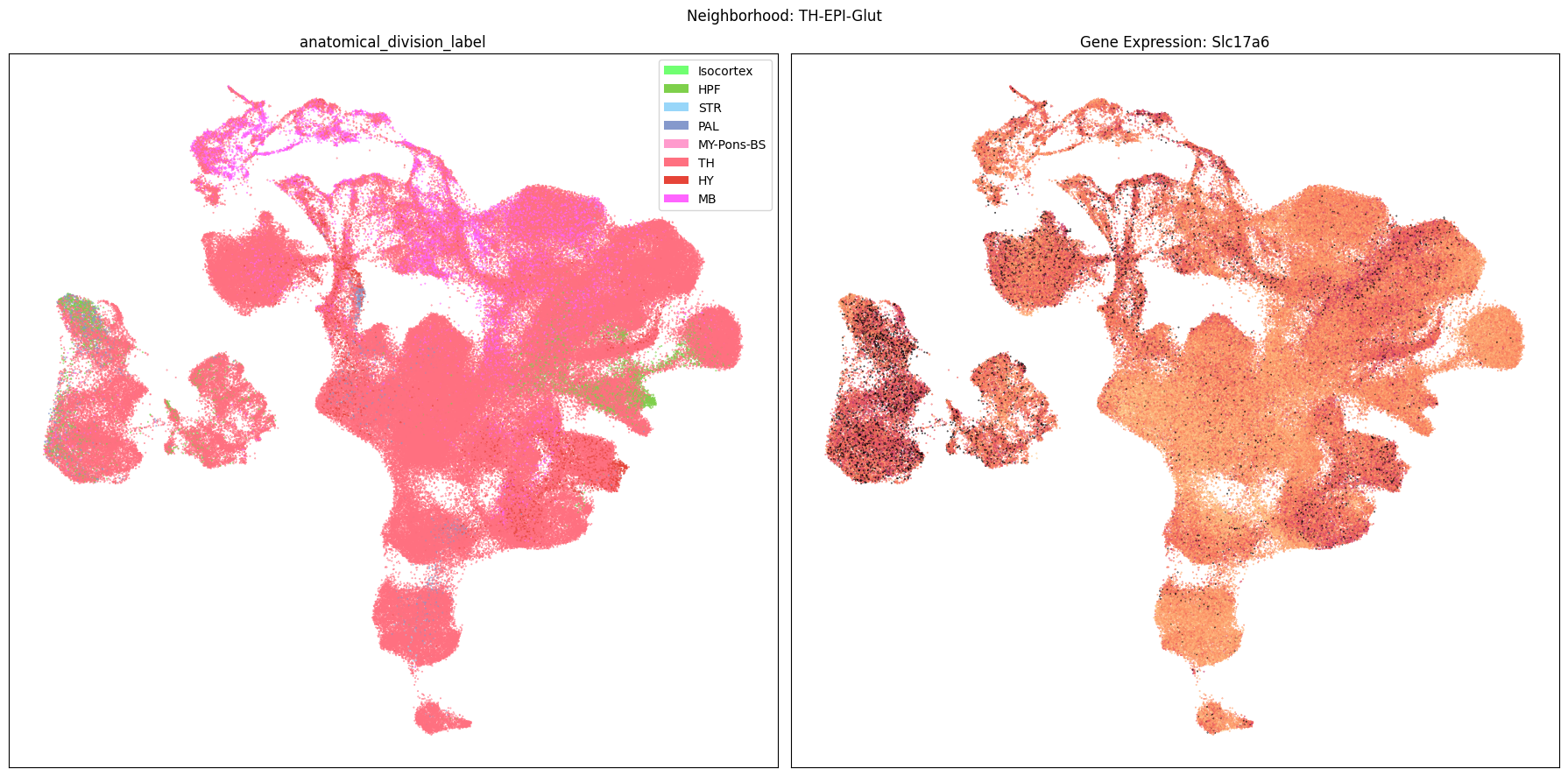
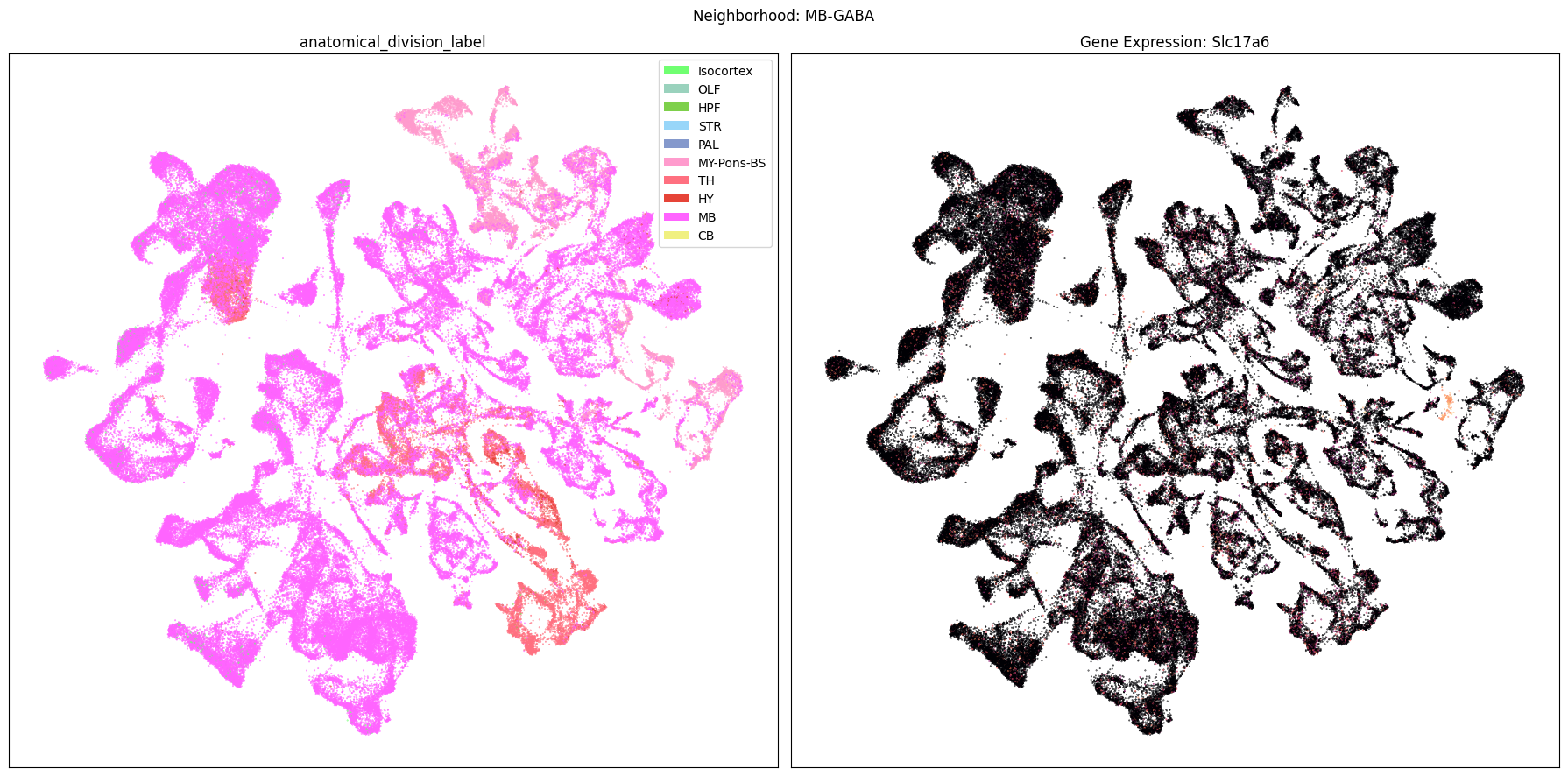
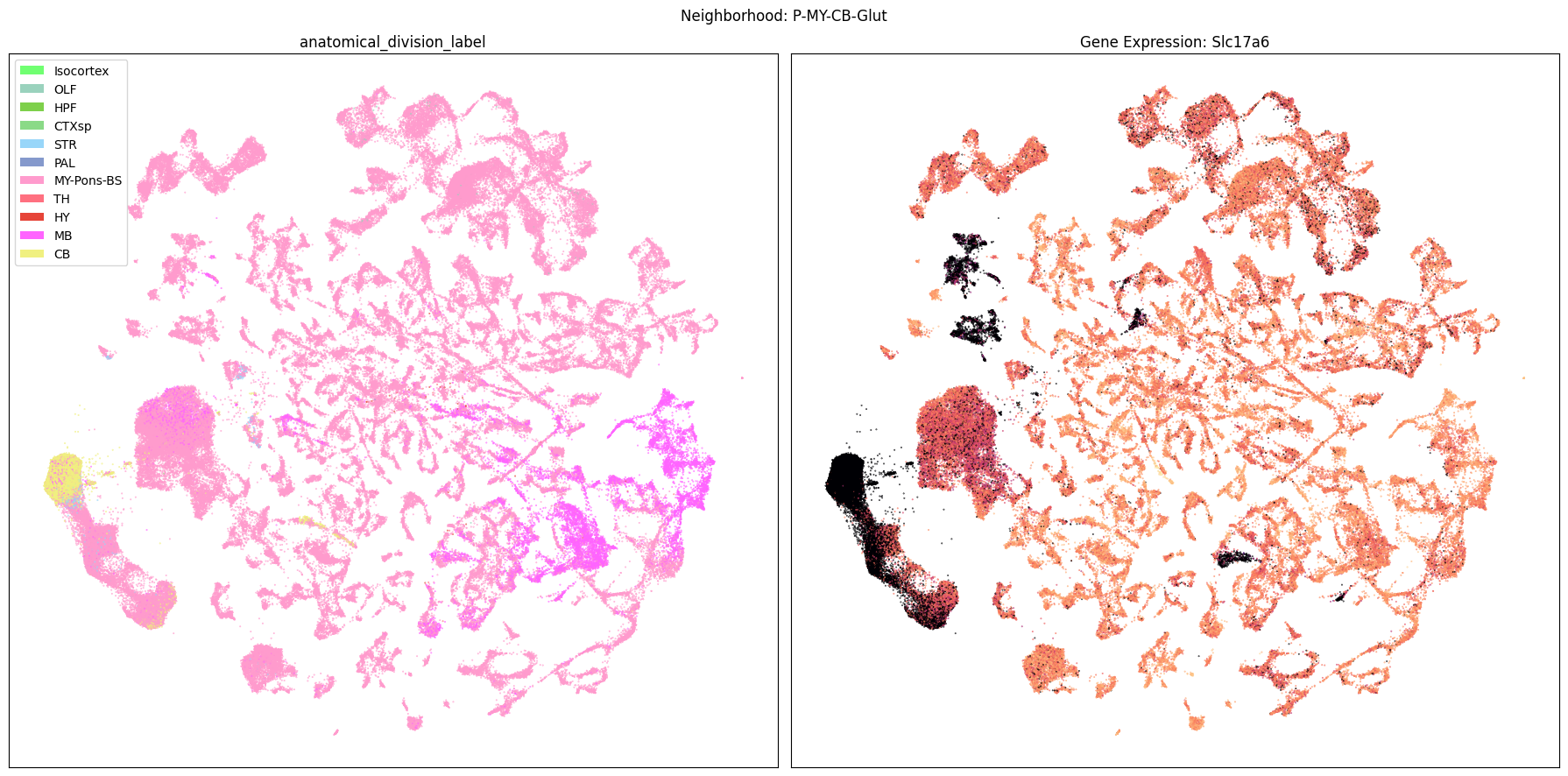
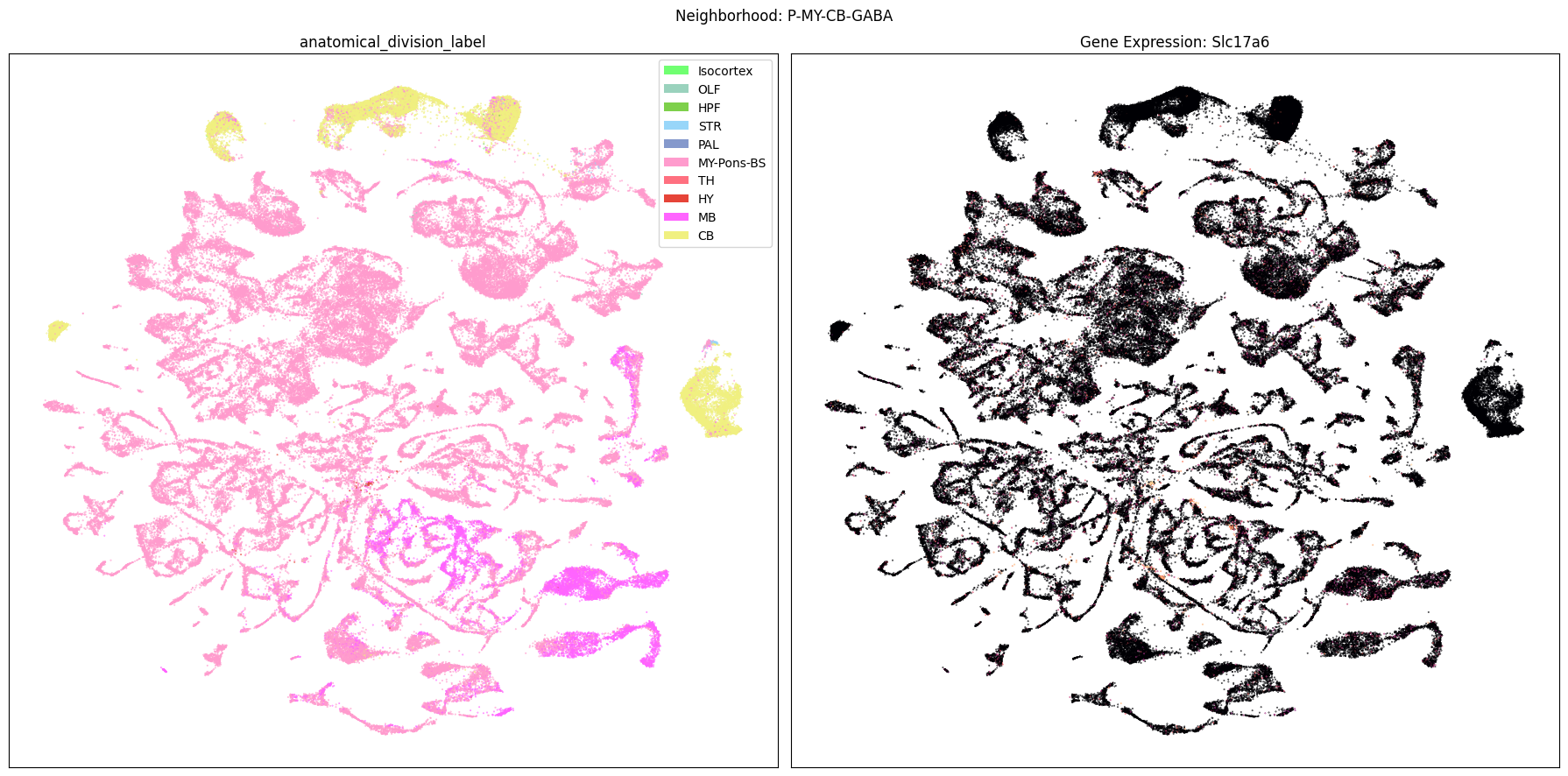
Finally, let’s plot each the anatomical regions and one of the genes, in this case we choose Slc17a6, for each of the 9 neighborhood UMAPs. Again, term_to_plot can be modified for to show e.g class, donor_sex, or any of the other data features with color and order information. gene_to_plot can be modified here to use any gene that was previously merged into our metadata tables.
term_to_plot = 'anatomical_division_label' # Can change to other metadata feature (e.g. taxonomy level)
sub_cell_extended = consensus_extended[::10]
gene_to_plot = gene_names[1]
for idx, neighborhood in enumerate(cluster_annotation_term[
cluster_annotation_term['cluster_annotation_term_set_name'] == 'neighborhood'
].sort_values('term_order')['name']):
# Loop through all neighborhoods and plot subclass UMAPs ordered by term_order.
fig, ax = plt.subplots(1, 2)
fig.set_size_inches(18, 9)
ax = ax.flatten()
neighborhood_cells = consensus_extended.join(
abc_cache.get_metadata_dataframe(
'Consensus-WMB-integrated-taxonomy',
f'{neighborhood}_cell_2d_embedding_coordinates'
).set_index('cell_label'),
how='inner',
rsuffix=f'_{neighborhood}'
)
plot_umap(
neighborhood_cells[f'x_{neighborhood}'],
neighborhood_cells[f'y_{neighborhood}'],
cc=neighborhood_cells[term_to_plot + '_color'],
labels=neighborhood_cells[term_to_plot],
term_orders=neighborhood_cells[term_to_plot + '_order'],
fig=fig,
ax=ax[0],
)
ax[0].set_title(term_to_plot)
plot_umap(
neighborhood_cells[f'x_{neighborhood}'],
neighborhood_cells[f'y_{neighborhood}'],
val=neighborhood_cells[gene_to_plot],
cmap=plt.cm.magma,
fig=fig,
ax=ax[1],
colorbar=False,
)
ax[1].set_title(f'Gene Expression: {gene_to_plot}')
fig.suptitle(f'Neighborhood: {neighborhood}')
plt.tight_layout()
plt.show()

Check out the previous exploration of the taxonomy and clustering annotations here. The notebooks for the AIBS, Whole Mouse Brain dataset can be found here.
