Statistically Testing 2P Responses to Stimulus#
In most analyses, some form of inclusion criteria is used to select neurons that are “responsive” to the stimulus conditions presented. There are no universally agreed upon inclusion criteria for this type of selection. In [Mesa et al., 2021], it is demonstrated that the choice of inclusion criteria can dramatically affect what neurons are selected as responsive. This notebook does a similar demonstration, using the same five inclusion criteria on Ophys dF/F recordings to select responsive neurons from one experimental session. It can be seen that very different selections are made depending on the criteria used. This also underscores how different criteria might be more or less appropriate for the type of stimulus and the type of measurements being used for analysis. We are sharing these so that this comparison can be reproduced on data and so researchers can more effectively use this choice to make their results more robust. For more information about dF/F data, see Visualizing 2P Responses to Stimulus.
Environment Setup#
⚠️Note: If running on a new environment, run this cell once and then restart the kernel⚠️
import warnings
warnings.filterwarnings('ignore')
try:
from databook_utils.dandi_utils import dandi_download_open
except:
!git clone https://github.com/AllenInstitute/openscope_databook.git
%cd openscope_databook
%pip install -e .
import matplotlib as mpl
import matplotlib.pyplot as plt
import numpy as np
import pandas as pd
from scipy import interpolate
from scipy.io import savemat
from scipy.stats import ttest_ind
%matplotlib inline
Downloading Ophys File#
Change the values below to download the file you’re interested in. In this example, we use the dF/F trace from an Ophys NWB File, so you’ll have to choose one with the same kind of data. Set dandiset_id and dandi_filepath to correspond to the dandiset id and filepath of the file you want. If you’re accessing an embargoed dataset, set dandi_api_key to your DANDI API key.
dandiset_id = "000036"
dandi_filepath = "sub-389014/sub-389014_ses-20180705T152908_behavior+image+ophys.nwb"
download_loc = "."
dandi_api_key = None
io = dandi_download_open(dandiset_id, dandi_filepath, download_loc, dandi_api_key=dandi_api_key)
nwb = io.read()
A newer version (0.62.1) of dandi/dandi-cli is available. You are using 0.61.2
File already exists
Opening file
Getting 2P Data and Stimulus Data#
Below, the fluorescence traces and timestamps are read from the file’s Processing section. In this notebook, we will be interested in the dF/F trace specifically. Note that the exact format to access these traces can vary between newer and older NWB files, so some adjustments may be necessary. Additionally, the stimulus data is also read from the NWB file’s Intervals section. Stimulus information is stored as a series of tables depending on the type of stimulus shown in the session. One such table is displayed below, as well as the names of all the tables. For more information on the dF/F data, see Visualizing 2P Responses to Stimulus.
# dff = nwb.processing["ophys"]["dff"]
# dff_trace = dff.roi_response_series["traces"].data
# dff_timestamps = dff.roi_response_series["traces"].timestamps
# you make have to change some of these keys
dff = nwb.processing["ophys"]["DfOverF"]
# transpose and cut out last element because this file is improperly formatted
dff_trace = np.array(dff.roi_response_series["imaging_plane_1"].data).transpose()
dff_timestamps = dff.roi_response_series["imaging_plane_1"].timestamps[:-1]
# accessing the above data may look different for older nwb files, like the following
# dff_trace = dff.roi_response_series["RoiResponseSeries"].data
# dff_timestamps = dff.roi_response_series["RoiResponseSeries"].timestamps
print(dff_trace.shape)
print(dff_timestamps.shape)
(127177, 41)
(127177,)
Fluorescence Interpolation#
Because we cannot be certain that the dF/F trace is acquired a perfectly regular sampling rate, we will interpolate it to be able to compare stimulus timestamps to their approximate dF/F times. After you have a valid list of timestamps, you can generate a linearly-spaced timestamp array called time_axis, and interpolate the dF/F data along it, making interpolated DFF data called interp_dff. This should be a 2D array with dimensions roi and time, where ROIs are the different cells whose fluorescence is measured. Here, the timestamps are interpolated to 10 Hertz, but you can change this by setting interp_hz.
interp_hz = 10
print(dff_trace.shape)
print(dff_timestamps.shape)
(127177, 41)
(127177,)
# generate regularly-space x values and interpolate along it
time_axis = np.arange(dff_timestamps[0], dff_timestamps[-1], step=(1/interp_hz))
interp_dff = []
# interpolate channel by channel to save RAM
for channel in range(dff_trace.shape[1]):
f = interpolate.interp1d(dff_timestamps, dff_trace[:,channel], axis=0, kind="nearest", fill_value="extrapolate")
interp_dff.append(f(time_axis))
interp_dff = np.array(interp_dff)
print(interp_dff.shape)
(41, 42181)
Selecting Stimulus Times#
Different types of stimulus require different kinds of inclusion criteria. Since the available stimulus tables vary significantly depending which NWB file and which experimental session you’re analyzing, you’ll have to adjust some values below. First, select which stimulus table you want by changing the key used below in nwb.intervals. The list of stimulus table names is printed below to inform this choice. Additionally, you’ll have to modify the function stim_select to select the stimulus times you want to use. In this example, a natural movie of worms is the selected stimulus because natural movies typically evoke strong responses. The repeated frame 40 is chosen here as our selected stimulus time, somewhat arbitrarily.
stimulus_names = list(nwb.intervals.keys())
print(stimulus_names)
[]
# use this if nwb intervals section has no stim information
def stim_obj_to_table(nwb):
all_labeled_stim_timestamps = []
for stim_type, stim_obj in nwb.stimulus.items():
start_times = stim_obj.timestamps[:-1]
stop_times = stim_obj.timestamps[1:]
frames = stim_obj.data[:-1]
l = len(start_times)
labeled_timestamps = list(zip(start_times, stop_times, frames, [stim_type]*l))
all_labeled_stim_timestamps += labeled_timestamps
all_labeled_stim_timestamps.sort(key=lambda x: x[0])
stim_table = pd.DataFrame(all_labeled_stim_timestamps, columns=("start time", "stop time", "frame", "stimulus type"))
return stim_table
# if there's no stim table, generate one from the stimulus objects
if len(nwb.intervals) == 0:
stim_table = stim_obj_to_table(nwb)
# otherwise, select one from options printed above
# stim_table = nwb.intervals["movie_worms_fwd_presentations"]
# print(stim_table.colnames)
stim_table[:10]
| start time | stop time | frame | stimulus type | |
|---|---|---|---|---|
| 0 | 27.475720 | 27.492416 | 0 | spontaneous |
| 1 | 27.492416 | 27.509112 | 0 | spontaneous |
| 2 | 27.509112 | 27.525758 | 0 | spontaneous |
| 3 | 27.525758 | 27.542211 | 0 | spontaneous |
| 4 | 27.542211 | 27.559146 | 0 | spontaneous |
| 5 | 27.559146 | 27.575829 | 0 | spontaneous |
| 6 | 27.575829 | 27.592468 | 0 | spontaneous |
| 7 | 27.592468 | 27.609146 | 0 | spontaneous |
| 8 | 27.609146 | 27.625847 | 0 | spontaneous |
| 9 | 27.625847 | 27.642543 | 0 | spontaneous |
set(stim_table["stimulus type"])
{'conspecifics',
'crickets',
'dots',
'human_montage',
'man_writing',
'mouse_montage_1',
'mouse_montage_1_spatial_phase_scramble',
'mouse_montage_1_temporal_phase_scramble',
'mouse_montage_2',
'mousecam',
'mousecam_spatial_phase_scramble',
'noise',
'snake',
'spontaneous'}
### select start times from table that fit certain criteria here
# stim_select = lambda row: True
# stim_select = lambda row: float(row.frame) == 40
# all_stim_times = [float(stim_table[i].start_time) for i in range(len(stim_table)) if stim_select(stim_table[i])]
all_stim_times = []
for i in range(len(stim_table)):
if i == 0:
continue
if stim_table["stimulus type"][i] == "mousecam" and stim_table["frame"][i] == 10 and stim_table["frame"][i-1] != 10:
all_stim_times.append(stim_table["start time"][i])
len(all_stim_times)
10
Aligning Stimulus Response Time Windows#
Now that you have your interpolated dF/F data and the selected stimulus timestamps, you identify the windows of time in the dF/F data that exist around each stimulus event. Since the dF/F data have been interpolated, we can easily translate timestamps to indices within the LFP trace array. Set window_start_time to be a negative number, representing the seconds before the stimulus event and window_end_time to be number of seconds afterward. Then the windows array will be generated as a set of slices of the interp_dff trace by using interp_hz to convert seconds to array indices. The output of this, the windows array, should be a 3D array shape trials by rois by time. These windows can be used for our analysis, and can be used to apply and display the affects of our various inclusion criteria.
window_start_time = -2
window_end_time = 3
# validate window bounds
if window_start_time > 0:
raise ValueError("start time must be non-positive number")
if window_end_time <= 0:
raise ValueError("end time must be positive number")
# get event windows
windows = []
window_length = int((window_end_time-window_start_time) * interp_hz)
for stim_ts in all_stim_times:
# convert time to index
start_idx = int( (stim_ts + window_start_time - dff_timestamps[0]) * interp_hz )
end_idx = start_idx + window_length
# bounds checking
if start_idx < 0 or end_idx > interp_dff.shape[1]:
continue
windows.append(interp_dff[:,start_idx:end_idx])
if len(windows) == 0:
raise ValueError("There are no windows for these timestamps")
windows = np.array(windows) * 100 # x100 to convert values to dF/F percentage
print(windows.shape)
(10, 41, 50)
Defining Plotting Methods#
Here are a few methods to display the stimulus response windows. These will be used to display the ROIs selected by each inclusion criteria. show_dff_response can be used to show one stimulus response window (or an average window). show_many_responses will be used to show many windows alongside each other. You can use the resulting plots to compare how different inclusion criteria can affect what rois are selected as responsive neurons.
def show_dff_response(ax, dff, window_start_time, window_end_time, aspect="auto", vmin=None, vmax=None, yticklabels=[], skipticks=1, xlabel="Time (s)", ylabel="ROI", cbar=True, cbar_label=None):
if len(dff) == 0:
print("Input data has length 0; Nothing to display")
return
img = ax.imshow(dff, aspect=aspect, extent=[window_start_time, window_end_time, 0, len(dff)], vmin=vmin, vmax=vmax)
if cbar:
fig.colorbar(img, shrink=0.5, label=cbar_label)
ax.plot([0,0],[0, len(dff)], ":", color="white", linewidth=1.0)
if len(yticklabels) != 0:
ax.set_yticks(range(len(yticklabels)))
ax.set_yticklabels(yticklabels, fontsize=8)
n_ticks = len(yticklabels[::skipticks])
ax.yaxis.set_major_locator(plt.MaxNLocator(n_ticks))
ax.set_xlabel(xlabel)
ax.set_ylabel(ylabel)
def show_many_responses(windows, rows, cols, window_idxs=None, title=None, subplot_title="", xlabel=None, ylabel=None, cbar_label=None, vmin=0, vmax=100):
if window_idxs is None:
window_idxs = range(len(windows))
windows = windows[window_idxs]
# handle case with no input data
if len(windows) == 0:
print("Input data has length 0; Nothing to display")
return
# handle cases when there aren't enough windows for number of rows
if len(windows) < rows*cols:
rows = (len(windows) // cols) + 1
fig, axes = plt.subplots(rows, cols, figsize=(2*cols+2, 2*rows+2), layout="constrained")
# handle case when there's only one row
if len(axes.shape) == 1:
axes = axes.reshape((1, axes.shape[0]))
for i in range(rows*cols):
ax_row = int(i // cols)
ax_col = i % cols
ax = axes[ax_row][ax_col]
if i > len(windows)-1:
ax.set_visible(False)
continue
window = windows[i]
show_dff_response(ax, window, window_start_time, window_end_time, xlabel=xlabel, ylabel=ylabel, cbar=False, vmin=vmin, vmax=vmax)
ax.set_title(f"{subplot_title} {window_idxs[i]}")
if ax_row != rows-1:
ax.get_xaxis().set_visible(False)
if ax_col != 0:
ax.get_yaxis().set_visible(False)
fig.suptitle(title)
norm = mpl.colors.Normalize(vmin=vmin, vmax=vmax)
colorbar = fig.colorbar(mpl.cm.ScalarMappable(norm=norm), ax=axes, shrink=1.5/rows, label=cbar_label)
Viewing Stimulus Response Windows#
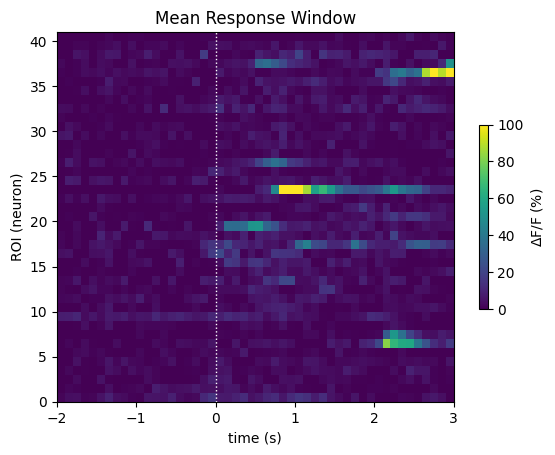
At last we are ready to play with the data. For starters, the stimulus windows can averaged across all stimulus trials to get an average view of how the ROIs respond.
mean_window = np.mean(windows, axis=0)
print(mean_window.shape)
fig, ax = plt.subplots()
show_dff_response(ax, mean_window, window_start_time, window_end_time, vmin=0, vmax=100, cbar_label="$\Delta$F/F (%)")
ax.set_title("Mean Response Window")
ax.set_xlabel("time (s)")
ax.set_ylabel("ROI (neuron)")
(41, 50)
Text(0, 0.5, 'ROI (neuron)')
Showing Response Windows#
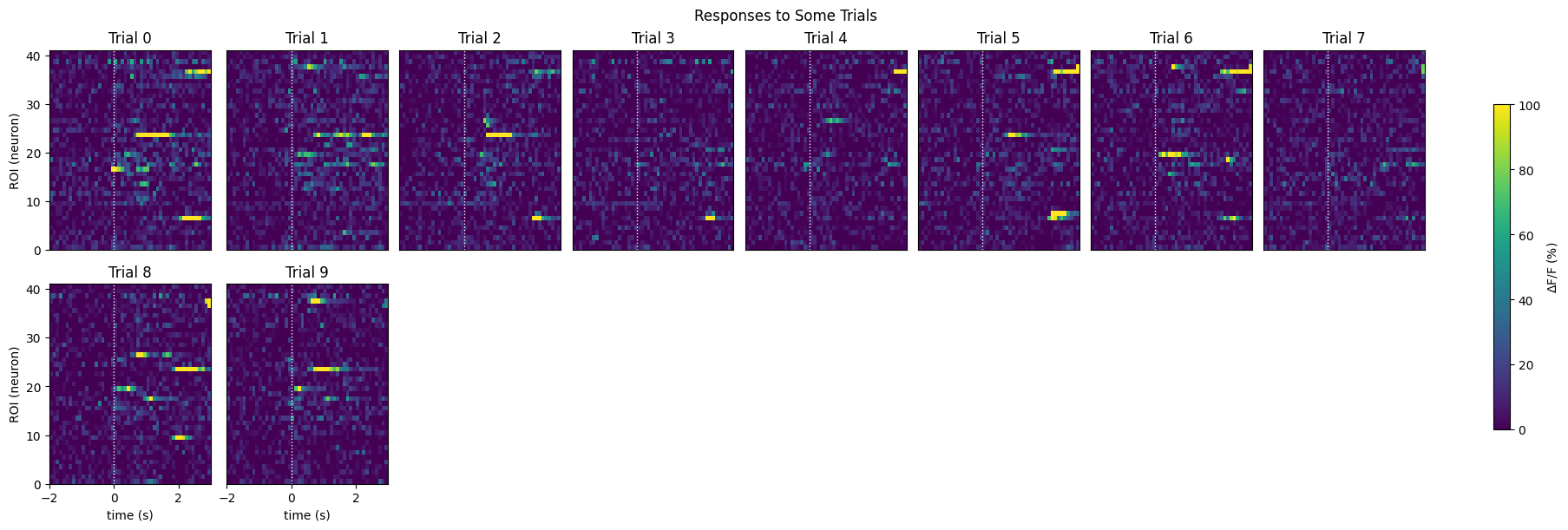
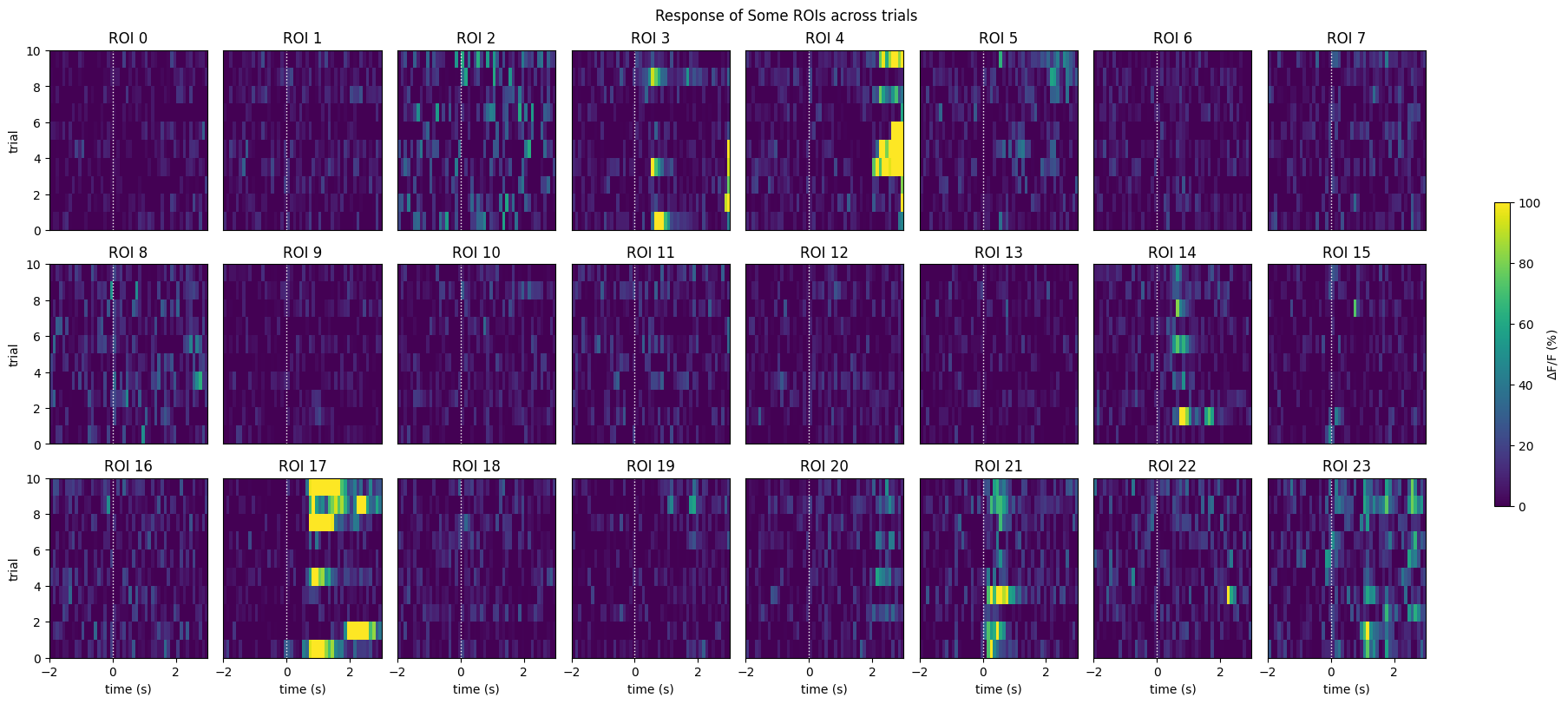
This is a view of many of the raw data response windows. Here, we show just the first 24 response windows. It can be seen that some neurons definitely show activity over time. However, it can be difficult to identify any patterns between trials with this view. Below, we also generate a neuronwise view of the first 24 neuron’s response windows. That is, a view of how each neuron responses across all trials. From this, we can see more clearly how neurons behave. Immediately, you can tell that some neurons have an evoked response at the onset of the stimulus, while others appear to be inhibited by the stimulus, and yet others show no strong responses.
show_many_responses(windows,
3,
8,
title="Responses to Some Trials",
subplot_title="Trial",
xlabel="time (s)",
ylabel="ROI (neuron)",
cbar_label="$\Delta$F/F (%)")
neuronwise_windows = np.swapaxes(windows,0,1)
show_many_responses(neuronwise_windows,
3,
8,
title="Response of Some ROIs across trials",
subplot_title="ROI",
xlabel="time (s)",
ylabel="trial",
cbar_label="$\Delta$F/F (%)")
Inclusion Criteria#
Now we can apply various inclusion criteria to see how they affect what neurons are selected. Five inclusion criteria are demonstrated below, but it should be noted that there are many other ways to select neurons, or to adjust the ones shown here. Different inclusion criteria have different levels of permissiveness, and will result in different behaviors in the selected cells. To start, we split the windows, in time, at the onset of the stimulus to yield the baseline activity of each roi, and the evoked_response activity. These should have shape trial by roi by time.
# get the index within the window that stimulus occurs (time 0)
stimulus_onset_idx = int(-window_start_time * interp_hz)
baseline = windows[:,:,0:stimulus_onset_idx]
evoked_responses = windows[:,:,stimulus_onset_idx:]
print(stimulus_onset_idx)
print(baseline.shape)
print(evoked_responses.shape)
20
(10, 41, 20)
(10, 41, 30)
Inclusion Criterion 1#
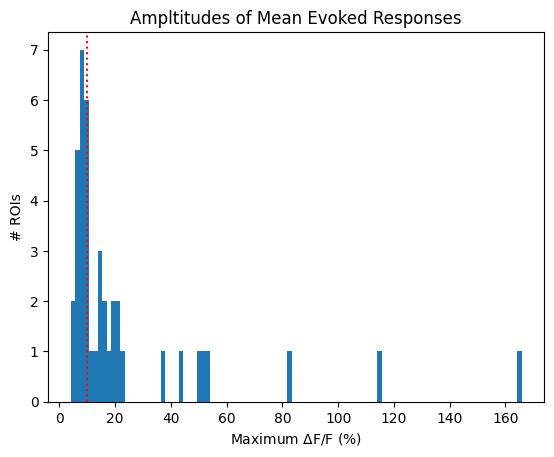
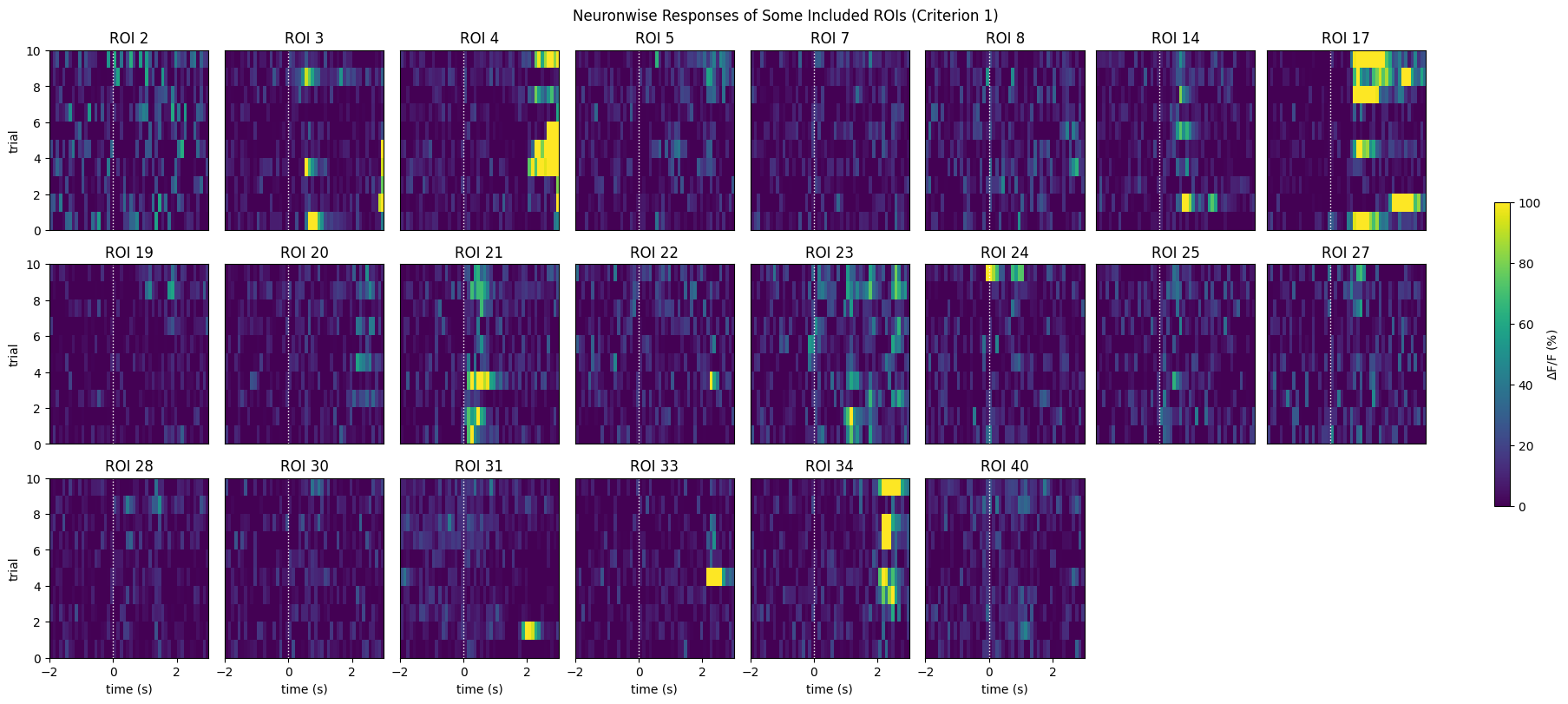
The first criterion is one of the simplest. This one is easy to alter and has a medium level of permissiveness. Below is shown a distribution of the ROI’s maximum mean evoked response. That is, the maximum dF/F value achieved during the evoked averaged response of an ROI to all stimulus trials. A line is drawn to show where the selection is made within the distribution. Also below are the neuronwise response windows of some of the selected neurons. It can be seen that these neurons appear more responsive than the (pseudo) random sample shown above.
The maximum value of the mean evoked response is >10% [Sun et al., 2016]
mean_evoked_responses = np.mean(evoked_responses, axis=0)
max_mean_evoked_responses = np.max(mean_evoked_responses, axis=1)
IC1_selected_rois = np.where(max_mean_evoked_responses > 10)[0]
print(f"Selected ROIs {IC1_selected_rois}")
Selected ROIs [ 2 3 4 5 7 8 14 17 19 20 21 22 23 24 25 27 28 30 31 33 34 40]
plt.hist(max_mean_evoked_responses, bins=100)
plt.ylabel("# ROIs")
plt.axvline(x=10, color="red", linestyle=":")
plt.xlabel("Maximum $\Delta$F/F (%)")
plt.title("Ampltitudes of Mean Evoked Responses")
plt.show()
print(f"{len(IC1_selected_rois)} / {neuronwise_windows.shape[0]} ROIs selected")
show_many_responses(neuronwise_windows,
3,
8,
window_idxs = IC1_selected_rois,
title="Neuronwise Responses of Some Included ROIs (Criterion 1)",
subplot_title="ROI",
xlabel="time (s)",
ylabel="trial",
cbar_label="$\Delta$F/F (%)")
22 / 41 ROIs selected
Inclusion Criterion 2#
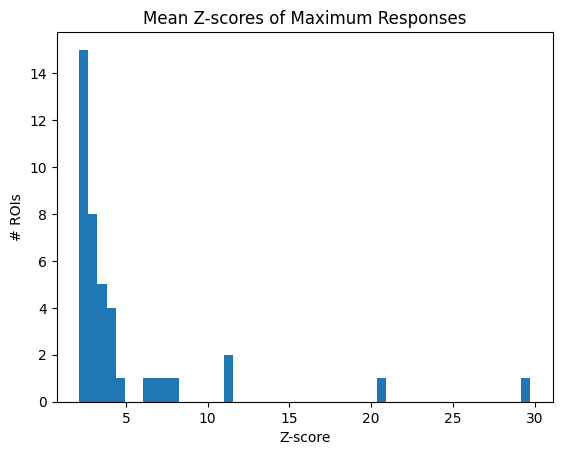
This criterion is slightly more complicated, and accounts for the fact that neurons might behave inconsistently. It uses the maximum values of the evoked response, and compares it to an absolute value (5%) as well as a value relative to its own baseline activity (3x the standard deviation). It is difficult to show the exact metric being used as a distribution, but to assist in interpretation, a distribution is shown below of the the mean ratio between the maximum evoked value and the standard distribution of the baseline behavior. Note that, this is not the exact metric used to select rois; as this criterion selects rois that exhibit significant activity in 50% or more trials. For our dataset, this criterion is rather stringent.
In 50% of trials, the evoked response is A) larger than 3x the SD of the baseline, and B) larger than 5% dF/F [Roth et al., 2012]
all_baseline_sds = np.std(baseline, axis=2)
max_responses = np.max(evoked_responses, axis=2)
# rois in each trial that respond more than 3x the SD of baseline
deviant_responses = max_responses > 3*all_baseline_sds
# rois in each trial that response more than 5% dff
large_responses = max_responses > 5
# rois in each trial that do both of the above
sig_responses = deviant_responses & large_responses
# rois that have significant responses in 50% or more trials
half_trials = sig_responses.shape[0] / 2
IC2_selected_rois = np.where( np.sum(sig_responses, axis=0) > half_trials )[0]
print(f"Selected ROIs {IC2_selected_rois}")
Selected ROIs [ 2 3 4 5 14 17 20 21 23 25 27 31 33 34 40]
z_scores = np.mean(max_responses / all_baseline_sds, axis=0)
plt.hist(z_scores, bins=50)
plt.xlabel("Z-score")
plt.ylabel("# ROIs")
# plt.axvline(x=3, color="red", linestyle=":")
plt.title("Mean Z-scores of Maximum Responses")
plt.show()
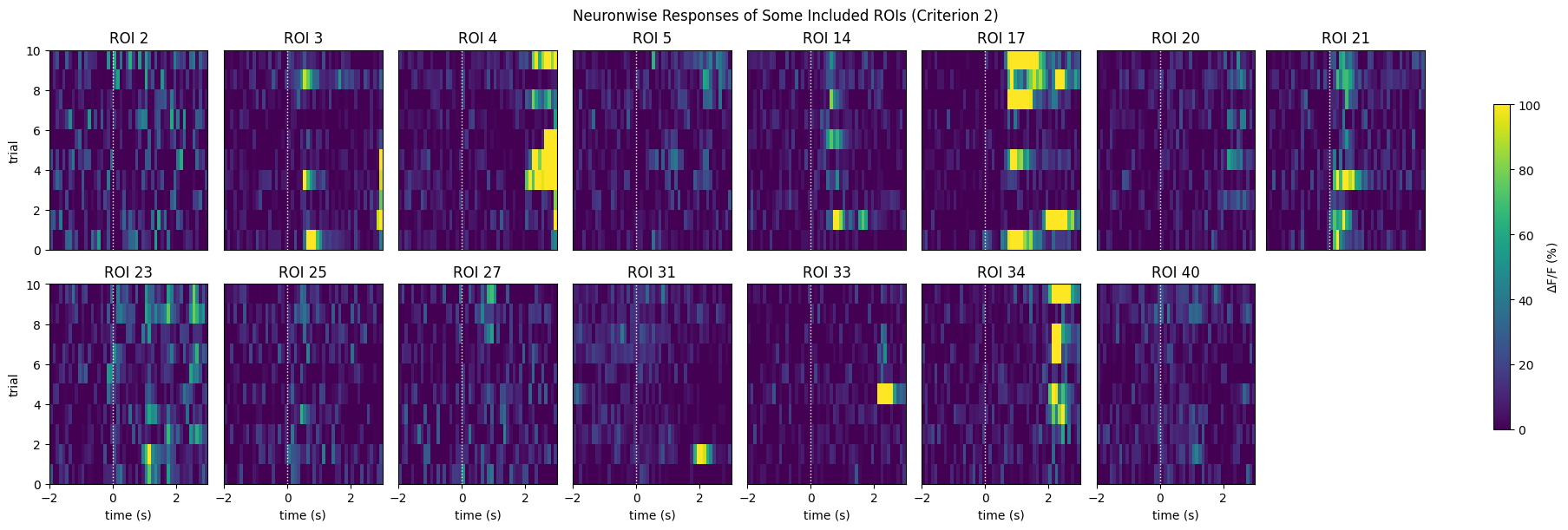
print(f"{len(IC2_selected_rois)} / {neuronwise_windows.shape[0]} ROIs selected")
show_many_responses(neuronwise_windows,
3,
8,
window_idxs=IC2_selected_rois,
title="Neuronwise Responses of Some Included ROIs (Criterion 2)",
subplot_title="ROI",
xlabel="time (s)",
ylabel="trial",
cbar_label="$\Delta$F/F (%)")
15 / 41 ROIs selected
Inclusion Criterion 3#
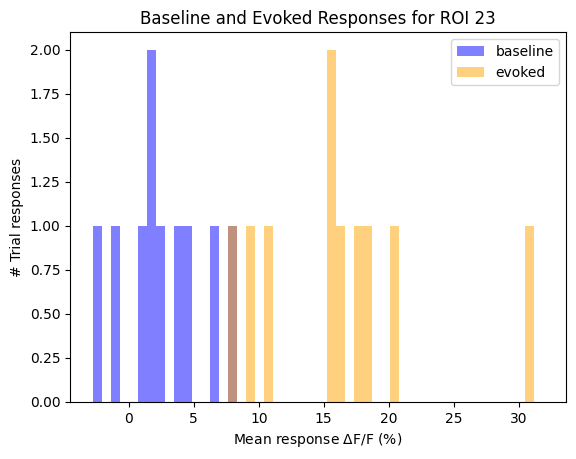
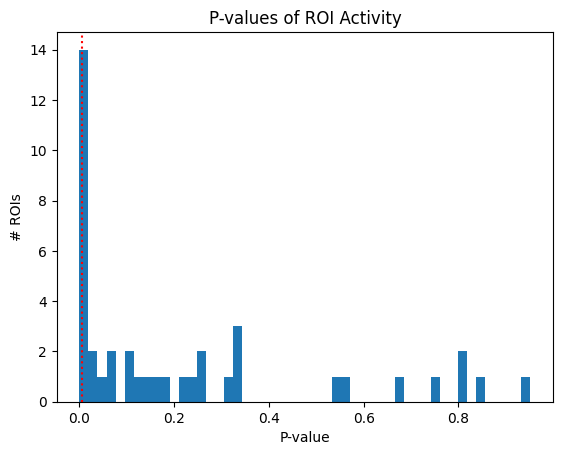
This is perhaps the most sophisticated criterion in our list. For each ROI, It compares the distribution of responses between the baseline activity and the evoked activity to identify any statistically significant response. Below, an example is shown of these distributions for one ROI. Change roi below to view the comparison for other cells. Additionally, the distribution of p-values of all rois is shown.
Paired T-test (p < 0.05) with Bonferroni correction, comparing the mean baseline to the mean evoked response [Andermann et al., 2011]
mean_trial_responses = np.mean(evoked_responses, axis=2)
mean_trial_baselines = np.mean(baseline, axis=2)
n = mean_trial_responses.shape[0]
t,p = ttest_ind(mean_trial_responses, mean_trial_baselines)
IC3_selected_rois = np.where(p < 0.05 / n)[0]
print(f"Selected ROIs {IC3_selected_rois}")
Selected ROIs [ 8 17 20 21 23 26]
roi = 21
baseline_dist = mean_trial_baselines[:,roi]
response_dist = mean_trial_responses[:,roi]
max_val = max(np.maximum(baseline_dist, response_dist)) + 1
min_val = min(np.minimum(baseline_dist, response_dist))
plt.hist(baseline_dist, bins=50, range=(min_val, max_val), alpha=0.5, color="blue", label="baseline")
plt.hist(response_dist, bins=50, range=(min_val, max_val), alpha=0.5, color="orange", label="evoked")
plt.legend(loc='upper right')
plt.xlabel("Mean response $\Delta$F/F (%)")
plt.ylabel("# Trial responses")
plt.title(f"Baseline and Evoked Responses for ROI {roi}")
plt.show
<function matplotlib.pyplot.show(close=None, block=None)>
hist, _, _ = plt.hist(p, bins=50)
plt.xlabel("P-value")
plt.ylabel("# ROIs")
thresh = 0.05/n
plt.axvline(x=thresh, color="red", linestyle=":")
plt.title("P-values of ROI Activity")
plt.show()
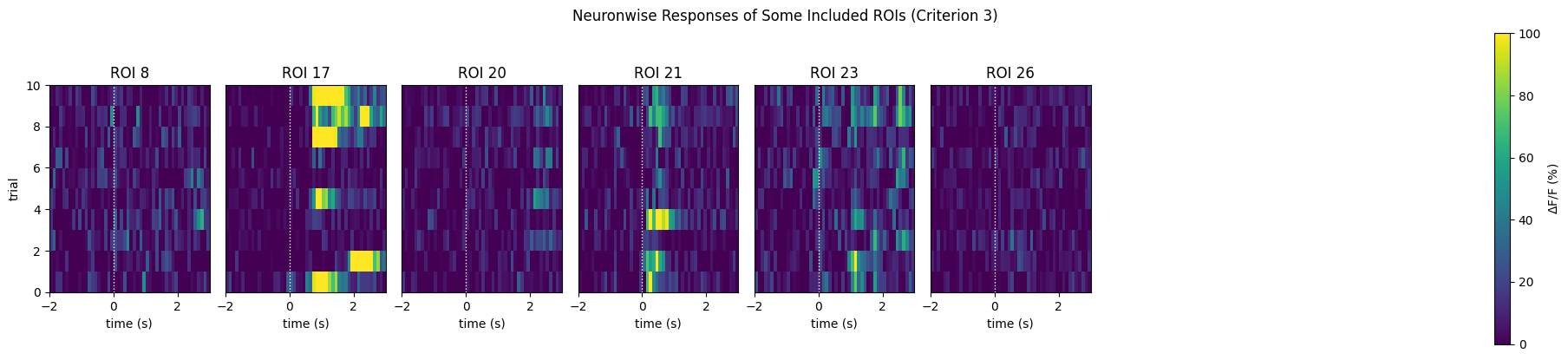
print(f"{len(IC3_selected_rois)} / {neuronwise_windows.shape[0]} ROIs selected")
show_many_responses(neuronwise_windows,
3,
8,
window_idxs=IC3_selected_rois,
title="Neuronwise Responses of Some Included ROIs (Criterion 3)",
subplot_title="ROI",
xlabel="time (s)",
ylabel="trial",
cbar_label="$\Delta$F/F (%)")
6 / 41 ROIs selected
Inclusion Criterion 4#
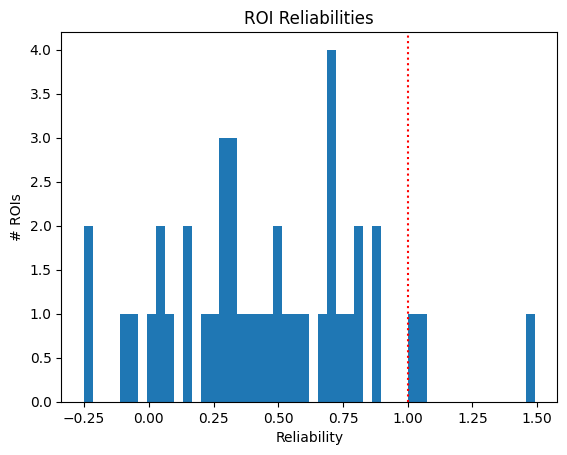
This criterion is the most stringent for our dataset. It relies on calculating the measure of reliability of a cell’s activity. In this example, 3 ROIs are selected.
A) The mean response to any stimulus condition is is > 6% dF/F and B) reliability > 1 [Marshel et al., 2012].
mean_trial_responses = np.mean(evoked_responses, axis=2)
response_means = np.mean(mean_trial_responses, axis=0)
response_sds = np.std(mean_trial_responses, axis=0)
mean_trial_baselines = np.mean(baseline, axis=2)
baseline_means = np.mean(mean_trial_baselines, axis=0)
baseline_sds = np.std(mean_trial_baselines, axis=0)
reliabilities = (response_means - baseline_means) / (response_sds + baseline_sds)
sig_reliabilities = reliabilities > 1
large_responses = response_means > 6
IC4_selected_rois = np.where(sig_reliabilities & large_responses)[0]
print(f"Selected ROIs {IC4_selected_rois}")
Selected ROIs [17 21 23]
plt.hist(reliabilities, bins=50)
plt.ylabel("# ROIs")
plt.xlabel("Reliability")
plt.axvline(x=1, color="red", linestyle=":")
plt.title("ROI Reliabilities")
plt.show()
print(f"{len(IC4_selected_rois)} / {neuronwise_windows.shape[0]} ROIs selected")
show_many_responses(neuronwise_windows,
3,
8,
window_idxs=IC4_selected_rois,
title="Neuronwise Responses of Some Included ROIs (Criterion 4)",
subplot_title="ROI",
xlabel="time (s)",
ylabel="trial",
cbar_label="$\Delta$F/F (%)")
3 / 41 ROIs selected

Inclusion Criterion 5#
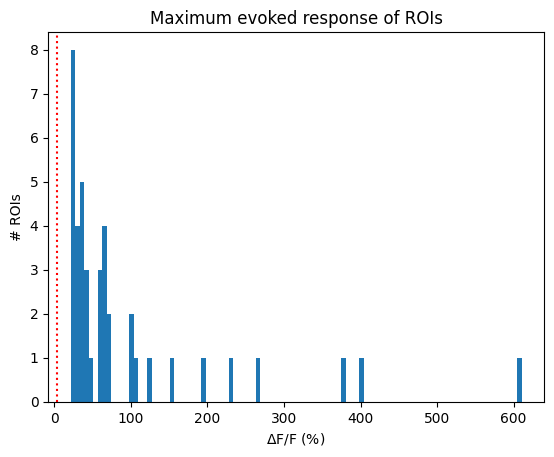
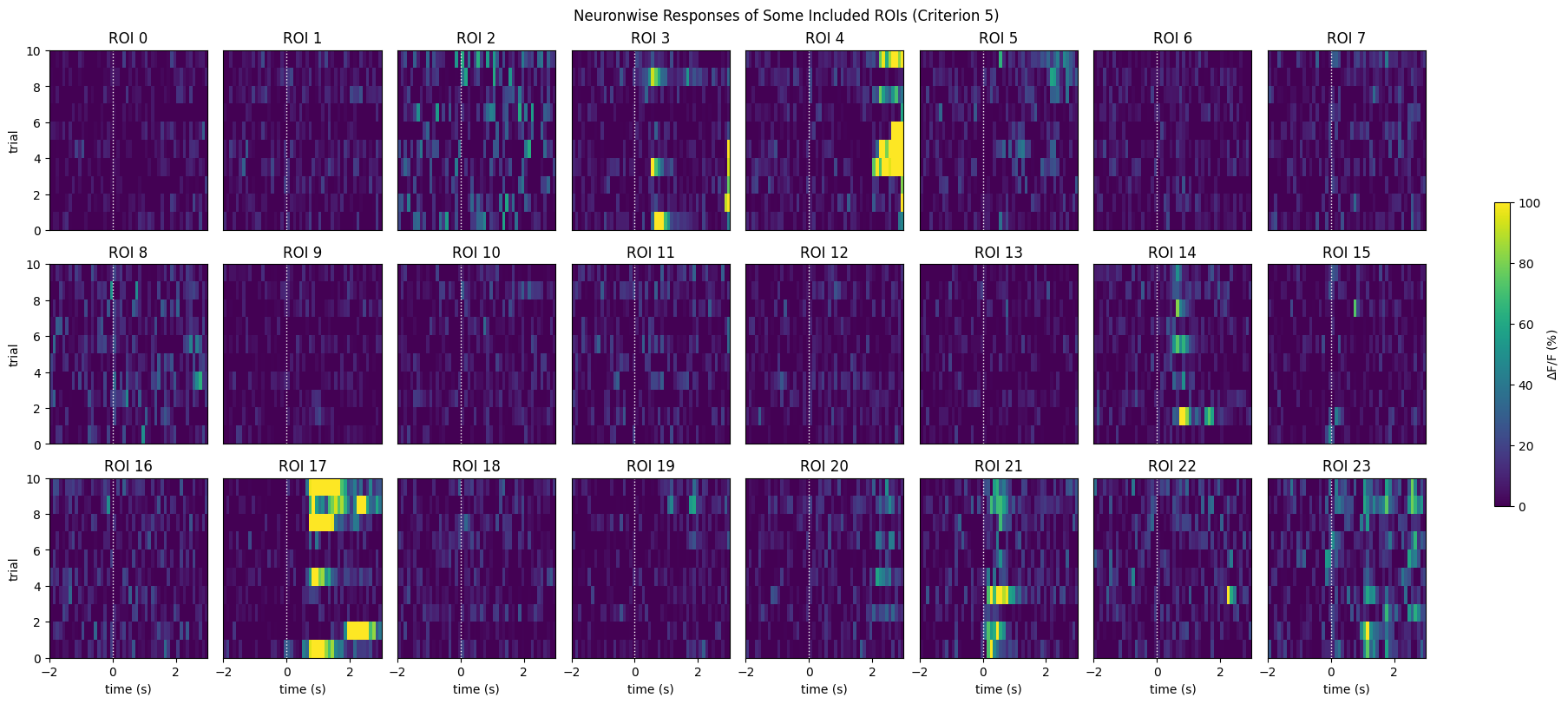
Finally, the simplest criterion. This criterion is clearly the most permissive, as it includes all ROIs for this dataset.
The maximum evoked response to any stimulus condition is > 4% dF/F [Tohmi et al., 2014].
max_responses = np.max(evoked_responses, axis=(0,2))
IC5_selected_rois = np.where(max_responses > 4)[0]
print(f"Selected ROIs {IC5_selected_rois}")
Selected ROIs [ 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40]
plt.hist(max_responses, bins=100)
plt.ylabel("# ROIs")
plt.xlabel("$\Delta$F/F (%)")
plt.axvline(x=4, color="red", linestyle=":")
plt.title("Maximum evoked response of ROIs")
plt.show()
print(f"{len(IC5_selected_rois)} / {neuronwise_windows.shape[0]} ROIs selected")
show_many_responses(neuronwise_windows,
3,
8,
window_idxs=IC5_selected_rois,
title="Neuronwise Responses of Some Included ROIs (Criterion 5)",
subplot_title="ROI",
xlabel="time (s)",
ylabel="trial",
cbar_label="$\Delta$F/F (%)")
41 / 41 ROIs selected
Saving Spike Matrix#
The fluorescence response windows can be saved/exported as a matrix for use in other programs using several methods. Below, numpy’s save method is used to save the matrix in the npy format for use in other Python programs. Scipy’s savemat function saves the matrix in a format usable for Matlab.
np.save("flr_matrix.npy", windows)
savemat("flr_matrix.mat", dict(flr_windows=windows))



